通过体内瞬时重编程扩展新皮质并防止神经变性
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
山中因子(YFs)可以逆转哺乳动物组织中的一些衰老特征,但它们对大脑的影响在很大程度上仍未被探索。在这里,我们在两种不同的情况下以时空可控的方式诱导了小鼠大脑中的YFs:大脑发育和神经退化背景下的成年阶段。胚胎期诱导 YFs 会扰乱祖细胞和神经元的细胞特性,但这些细胞能容忍瞬时和低水平的表达。在这些条件下,YF诱导导致祖细胞扩增、上皮层神经元和胶质细胞数量增加,并增强了成年小鼠的运动和社交行为。此外,在 5xFAD 小鼠模型中,成体背侧海马的主要神经元能耐受受控 YF 诱导,并能防止阿尔茨海默病的几个特征的发展,包括认知能力下降和分子特征改变。这些结果凸显了 YFs 对神经增殖的强大影响及其在脑部疾病中的潜在用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
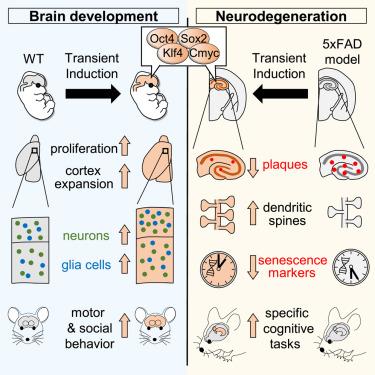
Expansion of the neocortex and protection from neurodegeneration by in vivo transient reprogramming
Yamanaka factors (YFs) can reverse some aging features in mammalian tissues, but their effects on the brain remain largely unexplored. Here, we induced YFs in the mouse brain in a controlled spatiotemporal manner in two different scenarios: brain development and adult stages in the context of neurodegeneration. Embryonic induction of YFs perturbed cell identity of both progenitors and neurons, but transient and low-level expression is tolerated by these cells. Under these conditions, YF induction led to progenitor expansion, an increased number of upper cortical neurons and glia, and enhanced motor and social behavior in adult mice. Additionally, controlled YF induction is tolerated by principal neurons in the adult dorsal hippocampus and prevented the development of several hallmarks of Alzheimer’s disease, including cognitive decline and altered molecular signatures, in the 5xFAD mouse model. These results highlight the powerful impact of YFs on neural proliferation and their potential use in brain disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


