半脊类顺式调控基因组学与中生代基因表达动力学
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
摘要
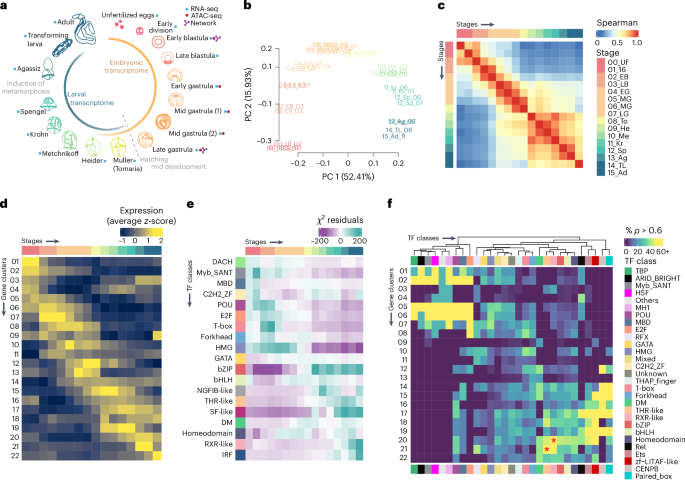
畸形动物是由半脊类和棘皮动物(统称为 Ambulacraria)以及脊索动物组成的双脊类的一个主要类群。这些类群之间的比较研究可以为了解畸齿目和双脊类最后共同祖先的性质提供有价值的信息。半脊索动物的间接发育与棘皮动物相似,其幼虫阶段与脊索动物和其他双脊类动物一样,具有前胸极性的成体平面,因此半脊索动物是研究中胚层动物发育的分子基础的合适模型。然而,目前仍缺乏有关半脊索动物基因表达和染色质动态的全面定量目录。在这项研究中,我们分析了间接发育半脊类动物 Ptychodera flava 多个发育阶段的转录组和染色质可及性。我们观察到,P. flava 的发育是由一个双相转录程序支撑的,该程序可能由不同的遗传网络控制。通过与其他双脊类动物的比较发现,在半脊索动物的胃形成期、头脊索动物的神经形成期和环带动物的伸长期,都存在类似的转录和调控动态。通过对调控网络的分析和棘皮动物转基因实验的功能验证,我们提出胃形成是中胚层动物分子相似性最高的阶段,中胚层动物发育的大部分分子基础可能存在于两翼动物的最后共同祖先中。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Hemichordate cis-regulatory genomics and the gene expression dynamics of deuterostomes
Deuterostomes are one major group of bilaterians composed by hemichordates and echinoderms (collectively called Ambulacraria) and chordates. Comparative studies between these groups can provide valuable insights into the nature of the last common ancestor of deuterostomes and that of bilaterians. Indirect development of hemichordates, with larval phases similar to echinoderms and an adult body plan with an anteroposterior polarity like chordates and other bilaterians, makes them a suitable model for studying the molecular basis of development among deuterostomes. However, a comprehensive, quantitative catalogue of gene expression and chromatin dynamics in hemichordates is still lacking. In this study, we analysed the transcriptomes and chromatin accessibility of multiple developmental stages of the indirect-developing hemichordate Ptychodera flava. We observed that P. flava development is underpinned by a biphasic transcriptional program probably controlled by distinct genetic networks. Comparisons with other bilaterian species revealed similar transcriptional and regulatory dynamics during hemichordate gastrulation, cephalochordate neurulation and elongation stages of annelids. By means of regulatory networks analysis and functional validations by transgenesis experiments in echinoderms, we propose that gastrulation is the stage of highest molecular resemblance in deuterostomes and that much of the molecular basis of deuterostome development was probably present in the bilaterian last common ancestor. Analysis of transcriptomes and chromatin accessibility of multiple developmental stages of the indirect-developing hemichordate Ptychodera flava shows similar transcriptional dynamics of different developmental stages across hemichordates, echinoderms and chordates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


