不依赖多聚腺苷酸的 RNA 聚合酶 II 终止机制
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
人们对 RNA 聚合酶 II(Pol II)转录的起始和延伸机制进行了深入研究,但对其终止机制的了解仍然很少。在这里,我们分析了由酵母 Sen1 螺旋酶介导的、不依赖于多腺苷酸的 Pol II 终止机制。两个终止前中间产物的冷冻电镜结构显示,Sen1 与 Pol II 结合,并利用其腺苷三磷酸酶活性沿 5′方向拉动流出的 RNA。据预测,这将推动 Pol II 向前移动,诱发不稳定的高转移状态,破坏转录泡的稳定性,从而促进转录终止。这种转录终止机制可能会被广泛使用,因为它在细菌转录系统中概念上是一致的。本文章由计算机程序翻译,如有差异,请以英文原文为准。

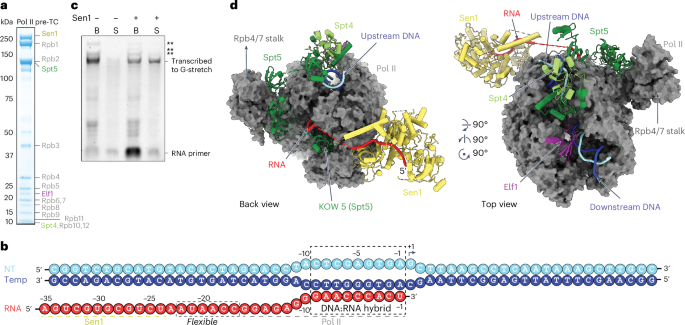
Mechanism of polyadenylation-independent RNA polymerase II termination
The mechanisms underlying the initiation and elongation of RNA polymerase II (Pol II) transcription are well-studied, whereas termination remains poorly understood. Here we analyze the mechanism of polyadenylation-independent Pol II termination mediated by the yeast Sen1 helicase. Cryo-electron microscopy structures of two pretermination intermediates show that Sen1 binds to Pol II and uses its adenosine triphosphatase activity to pull on exiting RNA in the 5′ direction. This is predicted to push Pol II forward, induce an unstable hypertranslocated state and destabilize the transcription bubble, thereby facilitating termination. This mechanism of transcription termination may be widely used because it is conceptually conserved in the bacterial transcription system. In this work, the authors report how the termination factor Sen1 interacts with an elongating RNA polymerase II (Pol II) and its nascent transcript to perform termination. Comparison of two pretermination states of Pol II supports a hypertranslocation model of termination.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


