用溴(二氟)乙酸获取结构多样的芳基二氟甲基醚
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们报告了一种使用廉价溴(二氟)乙酸对无处不在的酚类进行二氟甲基化的简化新方法,只需一步就能获得一系列结构多样的基于 OCF2H 的构筑基块。该方法使用现成的试剂,具有广泛的官能团兼容性,可用于有效的后期多样化和木质素衍生单体,这在之前的研究中尚未得到探索。本文章由计算机程序翻译,如有差异,请以英文原文为准。
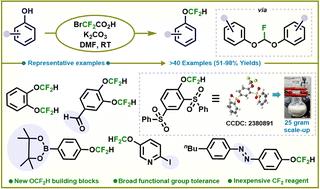
Accessing structurally diverse aryl difluoromethyl ethers with bromo(difluoro)acetic acid†
We report a new streamlined approach for the difluoromethylation of ubiquitous phenols using inexpensive bromo(difluoro)acetic acid, yielding a wide array of structurally diverse OCF2H-based building blocks in a single step. This method displays broad functional group compatibility and is amenable for effective late-stage diversification and lignin-derived monomers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


