依赖于SIRT7的乙酰化开关通过Pax5调控早期B细胞的分化和系承
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
B 淋巴造血是由系特异性转录因子协调的。在 B 细胞祖细胞中,系的承诺由 Pax5 介导,在 B 细胞急性淋巴细胞白血病中,Pax5 通常发生突变。尽管 Pax5 在免疫中发挥着重要作用,但其功能调控机制在很大程度上仍不为人所知。在这里,我们发现 NAD+依赖性酶 SIRT7 通过在 K198 处对 Pax5 进行去乙酰化来协调 B 细胞的发育,从而促进 Pax5 蛋白的稳定性和转录活性。Pax5K198去乙酰化或乙酰化模拟物都不能挽救Pax5-/-原B细胞的B细胞分化,这表明B细胞的发育需要Pax5动态去乙酰化。Pax5K198去乙酰化模拟物恢复了Pax5-/-原B细胞的系承诺和Sirt7-/-原B细胞的B细胞分化,表明分化与系承诺脱钩。SIRT7-Pax5的相互作用在B细胞急性淋巴细胞白血病中得到了保留,SIRT7的表达与预后良好相关。我们的研究结果揭示了B淋巴细胞生成的关键机制,并强调了sirtuins在免疫功能中的相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

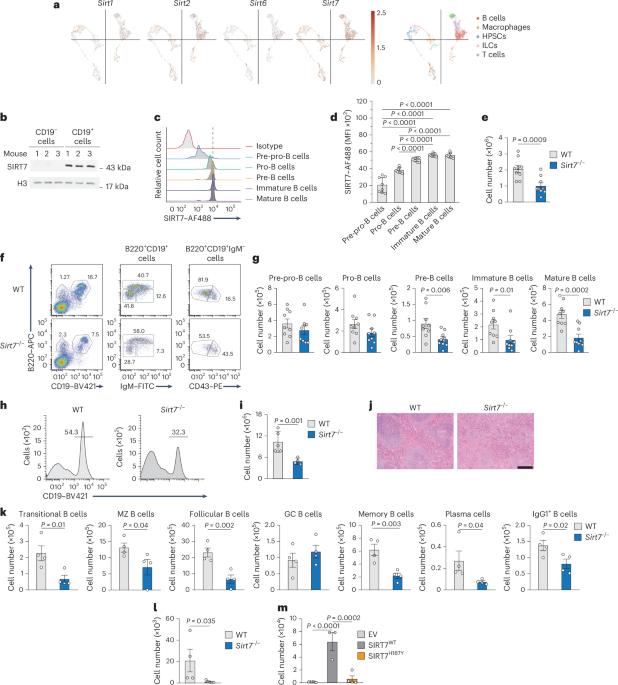
A SIRT7-dependent acetylation switch regulates early B cell differentiation and lineage commitment through Pax5
B lymphopoiesis is orchestrated by lineage-specific transcription factors. In B cell progenitors, lineage commitment is mediated by Pax5, which is commonly mutated in B cell acute lymphoblastic leukemia. Despite its essential role in immunity, the mechanisms regulating Pax5 function remain largely unknown. Here, we found that the NAD+-dependent enzyme SIRT7 coordinates B cell development through deacetylation of Pax5 at K198, which promotes Pax5 protein stability and transcriptional activity. Neither Pax5K198 deacetylated nor acetylated mimics rescued B cell differentiation in Pax5−/− pro-B cells, suggesting that B cell development requires Pax5 dynamic deacetylation. The Pax5K198 deacetylation mimic restored lineage commitment in Pax5−/− pro-B cells and B cell differentiation in Sirt7−/− pro-B cells, suggesting the uncoupling of differentiation from lineage commitment. The SIRT7–Pax5 interplay was conserved in B cell acute lymphoblastic leukemia, where SIRT7 expression correlated with good prognosis. Our findings reveal a crucial mechanism for B lymphopoiesis and highlight the relevance of sirtuins in immune function. Gámez-García et al. show that the deacetylase SIRT7 modulates the acetylation of Pax5 and its ability to repress alternate lineage programs and promote B cell differentiation and commitment in B cell progenitor cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


