紫外线诱导的含二酮烯醇的富电子吡咯环的 E/Z 异构化:理论和实验研究
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
2-acetyl-1,3-indandione, dehydroacetic acid, 3-acetyl-4-hydroxy-coumarin and acetyl-barbituric acid 与 pyrrol-2-carbaldehyde 的 Knoevenagel 反应生成 2-(1-hydroxy-3-(1H-pyrrol-2-yl)allylidene)-1H-indene-1,3(2H)-dione 5、3-(3-(1H-pyrrol-2-yl)acryloyl)-4-hydroxy-6-methyl-2H-pyran-2-one 6, 3-(3-(1H-pyrrol-2-yl)acryloyl)-4-hydroxy-2H-chromen-2-one 7 and 5-(1-hydroxy-3-(1H-pyrrol-2-yl)allylidene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione 8.通过核磁共振、红外光谱、紫外光谱和理论计算研究了紫外线诱导的 E→Z 异构化、分子内和分子间 H 键作用。E→Z 异构化的驱动力是在后者中形成一个分叉的 O-H⋯O⋯H-N 氢键。E⇆Z 平衡的位置在很大程度上取决于溶剂(CH2Cl2、丙酮、EtOH、DMSO)。根据 QTAIM 分析,形成的 OH⋯O 键能量较强或中等(从 7.6 到 10.2 kcal/mol),而 NH⋯O 键能量较弱(从 4.8 到 6.4 kcal/mol)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
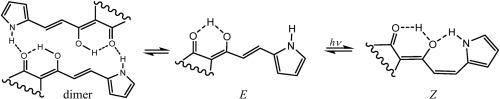
UV-induced E/Z isomerization of the electron-rich pyrrole ring containing diketoenols: Theoretical and experimental study
The Knoevenagel reaction of 2-acetyl-1,3-indandione, dehydroacetic acid, 3-acetyl-4-hydroxy-coumarin and acetyl-barbituric acid with pyrrol-2-carbaldehyde affords 2-(1-hydroxy-3-(1H-pyrrol-2-yl)allylidene)-1H-indene-1,3(2H)-dione 5, 3-(3-(1H-pyrrol-2-yl)acryloyl)-4-hydroxy-6-methyl-2H-pyran-2-one 6, 3-(3-(1H-pyrrol-2-yl)acryloyl)-4-hydroxy-2H-chromen-2-one 7 and 5-(1-hydroxy-3-(1H-pyrrol-2-yl)allylidene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione 8. The UV-induced E→Z isomerization, intra and intermolecular H-bonding is studied by NMR, IR, UV spectroscopy and theoretical calculations. The driving force for the E→Z isomerization is the formation of a bifurcate O–H⋯O⋯H–N hydrogen bond in the latter. The position of the E⇆Z equilibrium strongly depends on the solvent (CH2Cl2, acetone, EtOH, DMSO). According to QTAIM analysis, the formed OH⋯O bonds are strong or moderate in energy (from 7.6 to 10.2 kcal/mol), while the NH⋯O bonds are weak (from 4.8 to 6.4 kcal/mol).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


