智能纳米催化剂介导的溶酶体消融途径协调肿瘤治疗的放大效应
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
活性氧(ROS)的产生易受外部激发或相关参与者(如过氧化氢(H2O2)和敏化剂)供应不足的影响,从而限制了 ROS 驱动的肿瘤治疗。此外,溶酶体滞留效应严重阻碍了基于 ROS 的纳米系统的利用,严重制约了肿瘤的治疗效果。因此,本文首次报道了一种智能纳米催化剂TCPP-Cu@MnOx((MnII)1(MnIII)2.1(MnIV)2.6O9.35),并提出了一种治疗肿瘤的程序化ROS放大策略。最初,酸性解锁纳米催化剂被主动触发,产生大量单线态氧(1O2),介导酸性溶酶体消融,以帮助纳米催化剂逃逸并部分诱导溶酶体死亡,这一阶段被称为溶酶体驱动疗法。更令人意想不到的是,与中性介质(pH 7.4)相比,酸性条件(pH 5.0)下的 1O2 产量更高,两者相差约 204 倍。随后,逸出的纳米催化剂进一步激活了 H2O2- 介导的 1O2 和羟基自由基(-OH)的生成以及谷胱甘肽(GSH)的消耗,从而进一步提高了肿瘤治疗的效率。因此,在该系统中,提出了一种可程序激活的 TCPP-Cu@MnOx 纳米催化剂,通过诱导 1O2 高效破坏细胞器-溶酶体,并刺激细胞质中的 H2O2 转化为高毒性的 1O2 和 -OH,为克服目前 ROS 治疗的局限性提供了一种有吸引力的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
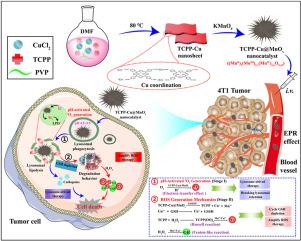
Intelligent nanocatalyst mediated lysosomal ablation pathway to coordinate the amplification of tumor treatment
The production of reactive oxygen species (ROS) is susceptible to external excitation or insufficient supply of related participants (e.g., hydrogen peroxide (H2O2) and sensitizer), liming ROS-driven tumor treatment. Additionally, the lysosomal retention effect severely hinders the utilization of ROS-based nanosystems and severely restricted the therapeutic effect of tumors. Therefore, first reported herein an intelligent nanocatalyst, TCPP-Cu@MnOx ((MnII)1(MnIII)2.1(MnIV)2.6O9.35), and proposed a programmed ROS amplification strategy to treat tumors. Initially, the acidity-unlocked nanocatalyst was voluntarily triggered to generate abundant singlet oxygen (1O2) to mediate acid lysosomal ablation to assist nanocatalyst escape and partially induce lysosomal death, a stage known as lysosome-driven therapy. More unexpectedly, the high-yielding production of 1O2 in acid condition (pH 5.0) was showed compared to neutral media (pH 7.4), with a difference of about 204 times between the two. Subsequently, the escaping nanocatalyst further activated H2O2-mediated 1O2 and hydroxyl radical (•OH) generation and glutathione (GSH) consumption for further accentuation tumor therapy efficiency, which is based on the Fenton-like reaction and Russell reaction mechanisms. Therefore, in this system, a program-activatable TCPP-Cu@MnOx nanocatalyst, was proposed to efficiently destruct organelle-lysosome via 1O2 inducing, and stimulated H2O2 conversion into highly toxic 1O2 and •OH in cytoplasm, constituting an attractive method to overcome limitations of current ROS treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


