磷酸化强烈影响对人碳酸酐酶 I CO2 水合活性的抑制作用
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
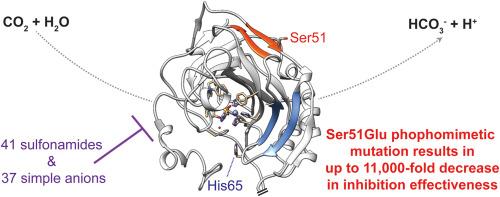
人类碳酸酐酶(hCAs)在呼吸、酸碱平衡和体液分泌中发挥着重要作用,对青光眼、癫痫、肥胖和癌症等疾病也有影响。在已知的 15 种 hCA 中,人 CA I(hCA I)在红细胞中的含量尤其丰富,在二氧化碳转运中发挥着关键作用。尽管对 hCA I 进行了广泛的研究,但人们对其翻译后修饰(PTM),尤其是磷酸化对其催化活性和抑制剂结合的影响仍然知之甚少。虽然通过高通量蛋白质组学研究发现了体内 hCA I 的多个磷酸化位点,包括高度保守的 Ser51 残基,但这些修饰的功能性后果还没有得到很好的描述。我们研究了 Ser51 磷酸化突变对 hCA I 的影响,考察了其催化效率以及对磺胺类药物和阴离子抑制的敏感性。利用重组表达系统和停流动力学测定,我们鉴定了 S51E hCA I 与野生型酶相比的二氧化碳水合活性和抑制曲线。我们的结果表明,S51E 突变将催化周转率(kcat)从 2.0 × 105 s-1 提高到 2.6 × 105 s-1,但却显著降低了底物亲和力,将迈克尔常数(KM)从 4.0 mM 提高到 13.9 mM,使总体催化效率降低了 50%以上。使用 41 种磺胺类药物进行的抑制研究表明,S51E 突变极大地改变了抑制剂的敏感性,尤其是对最有效的抑制剂。例如,在 16 种对 hCA I 最有效的磺胺类抑制剂(KIs 为 350 nM)中,有 15 种抑制 S51E hCA I 的效果比野生型平均低 35 倍以上。抗惊厥药唑尼沙胺的 KI 从野生型 hCA I 的 31 nM 增加到 4.0 μM。由 37 种小阴离子组成的抑制谱进一步表明,在 37 种测试阴离子中,S51E 突变体对其中 24 种阴离子的抑制敏感性显著降低,对于硫化氢等抑制剂,一些 KI 值增加了高达 11,000 倍。这项研究强调了磷酸化可能对 hCA I 的功能和抑制作用产生的重大影响。通过描述磷酸化对 hCA I 的二氧化碳水合活性和抑制剂敏感性的影响,这些发现代表了开发更具选择性的蛋白形式特异性抑制剂的早期步骤,这可能会为涉及碳酸酐酶的疾病带来更有效的治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Phosphorylation strongly affects the inhibition of human carbonic anhydrase I CO2 hydration activity
Human carbonic anhydrases (hCAs) have essential roles in respiration, acid-base balance, and fluid secretion, with implications in diseases such as glaucoma, epilepsy, obesity, and cancer. Of the fifteen known hCAs, human CA I (hCA I) is particularly abundant in erythrocytes, playing a critical role in CO2 transport. Despite extensive research on hCA I, the impact of post-translational modifications (PTMs), particularly phosphorylation, on its catalytic activity and inhibitor binding remains poorly understood. Although multiple phosphorylation sites have been identified in hCA I in vivo through high-throughput proteomics studies including at the highly conserved Ser51 residue, the functional consequences of these modifications are not well characterized. We investigated the effects of a phosphomimetic mutation at Ser51 on hCA I, examining its catalytic efficiency and susceptibility to inhibition by sulfonamides and anions. Using a recombinant expression system and a stopped-flow kinetic assay, we characterized the CO2 hydration activity and inhibition profiles of S51E hCA I compared to the wild type enzyme. Our results demonstrate that the S51E mutation increases the catalytic turnover rate (kcat) from 2.0 × 105 s−1 to 2.6 × 105 s−1 but significantly decreases substrate affinity, raising the Michaelis constant (KM) from 4.0 mM to 13.9 mM, reducing overall catalytic efficiency by over 50 %. Inhibition studies with a panel of 41 sulfonamides revealed that the S51E mutation dramatically alters inhibitor sensitivity, particularly for the most effective inhibitors. For example, 15 of the 16 most effective sulfonamide inhibitors for hCA I (with KIs <350 nM) were an average of over 35-fold less effective in inhibiting S51E hCA I than the wild type. The KI of the anticonvulsant zonisamide increased from 31 nM for the wild type hCA I to 4.0 μM. The inhibition profile with a panel of 37 small anions further indicated that the S51E mutant exhibited significantly reduced susceptibility to inhibition by 24 out of 37 tested anions, with some KI values increasing by up to 11,000-fold for inhibitors like hydrogen sulfide. This study underscores the significant impact that phosphorylation may have on hCA I function and inhibition. By characterizing the effects of phosphorylation on the CO2 hydration activity and inhibitor sensitivity of hCA I, these findings represent early steps in developing more selective proteoform-specific inhibitors, which could lead to more effective treatments for diseases involving carbonic anhydrases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


