戊乙酸/氨气协同稳定铜(II),作为硫代硫酸盐绿色浸金的高效氧化剂
IF 4.9
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
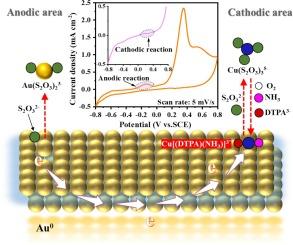
人们日益认识到,硫代硫酸盐浸出法是氰化法提取黄金的一种环境可持续替代方法。该领域的主要挑战之一是为 Cu(II)氧化剂找到一种有效的稳定剂,同时确保高效的金浸出和较低的硫代硫酸盐消耗。这项工作引入了戊乙酸(DTPA)作为铜(II)氧化剂的稳定剂,形成了一种基于稳定螯合 CuDTPA3- 复合物的新型氧化系统。使用 (NH4)2S2O3 和 Na2S2O3 进行的比较浸出凸显了氨(NH3)在增强稳定 CuDTPA3- 复合物氧化能力方面的有利作用。所提出的机理表明,NH3 有助于形成具有更强化学反应活性的混合[Cu(DTPA)(NH3)]3- 复合物。此外,DTPA 还能有效保持浸出系统的稳定性,从而提高金的浸出率、减少硫代硫酸盐的消耗并减轻钝化。通过精确的参数控制,预处理金精矿的金浸出效率高达 97.8%,(NH4)2S2O3 消耗量低(8.7 公斤/吨-孔),效果令人满意。此外,实际操作过程可以通过使用成本更低的浸出药剂(如添加少量 NH4OH 的 Na2S2O3 或各种铵盐,如 (NH4)2SO4、CH3COONH4 和 NH4Cl)来简化设计。这项研究为提高环保型金浸出工艺的商业可行性提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pentetic acid/ammonia cooperatively stabilizes Cu(II) as an efficient oxidant for green thiosulfate leaching of gold
Thiosulfate leaching is increasingly recognized as an environmentally sustainable alternative to cyanidation for extracting gold. One of the primary challenges in this field is identifying an effective stabilizer for the Cu(II) oxidant that simultaneously ensures efficient gold leaching and low thiosulfate consumption. This work introduces pentetic acid (DTPA) as a stabilizer for Cu(II) oxidant, forming a novel oxidation system based on the stable-chelated CuDTPA3− complex. Comparative leaching using (NH4)2S2O3 and Na2S2O3 highlight the beneficial role of ammonia (NH3) in enhancing the oxidation capability of the stable CuDTPA3− complex. The proposed mechanism suggests that NH3 contributes to the formation of a mixed [Cu(DTPA)(NH3)]3− complex with enhanced chemical reactivity. Additionally, DTPA is shown to effectively maintain the stability of the leaching system, thereby improving gold leaching, reducing thiosulfate consumption, and mitigating passivation. Through precise parameters control, a satisfactory gold leaching from a pretreated gold concentrate achieved a high efficiency of 97.8 %, with a low (NH4)2S2O3 consumption (8.7 kg/t-ore). Furthermore, the actual operation process can be facile designed by using more cost-effective leaching chemicals such as Na2S2O3 with a little addition of NH4OH or various ammonium salts, like (NH4)2SO4, CH3COONH4, and NH4Cl. This study provides valuable insights for advancing the commercial viability of environmentally friendly gold leaching processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


