通过催化调整原氨基苯酚的转化控制碳点的发射颜色和化学结构
IF 11.6
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
合成具有定制特性的碳点(CD)通常需要耗时的试错实验,部分原因是对前驱体材料的化学转化缺乏了解和控制。在此,我们首次报道了固态热解或溶解热转化邻氨基苯酚(oAP)前体的情况,邻氨基苯酚前体由苯环上正交分布的氨基和羟基组成。我们发现这两种转化反应都会产生两种发射颜色的产物,通过重复柱色谱法可将其分离为不同的蓝色发射光盘(bCDs,λ峰 = 420 nm)和黄色发射光盘(yCDs,λ峰 = 565 nm)。系统表征结果表明,这两种发光二极管都具有类似平面石墨烯的内部结构,但它们的区别在于,bCDs 包含一个混合的富含氨基的重要荧光团,而 yCDs 则包含一个富含吡啶的荧光团。这意味着 bCDs 是通过活化 oAP 前体的氨基形成的,而 yCDs 的合成则是同时活化氨基和羟基。有了这些知识,我们就可以通过添加催化剂(路易斯酸 AlCl3-6H2O 或路易斯碱 NaOH)来引导 oAP 前体的化学转化,从而只生成 bCDs 或 yCDs。该演示的重要意义在于它表明,可以通过高效合理的方法而不是反复试验的方法来合成具有所需性质的 CD。本文章由计算机程序翻译,如有差异,请以英文原文为准。
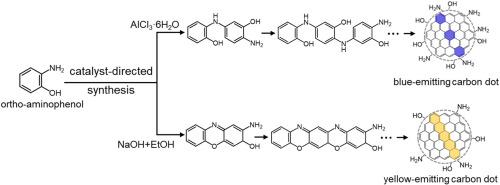
Controlling the emission colour and chemical structure of carbon dots by catalysis-tuned conversion of ortho-aminophenol
The synthesis of carbon dots (CDs) with tailored properties commonly requires time-consuming trial-and-error experimentation, in part because of a poorly understood and controlled chemical conversion of the precursor material. Here, we first report on the solid-state pyrolysis or solvothermal conversion of an ortho-aminophenol (oAP) precursor, comprising ortho-disposed amino and hydroxyl groups on a benzene ring. We find that both conversion reactions resulted in a two emission-colour product, which could be separated into distinct blue-emitting CDs (bCDs, λpeak = 420 nm) and yellow-emitting CDs (yCDs, λpeak = 565 nm) by repetitive column chromatography. Systematic characterization revealed that both CDs comprise a planar graphene-like interior, but that they are distinguished by that the bCDs comprise an intermixed significant amino-rich fluorophore while the yCDs instead comprise a pyridinic-rich fluorophore. This implies that the bCDs are formed via activation of the amino group of the oAP precursor, whereas the synthesis of the yCDs constituted a simultaneous activation of both the amino and hydroxyl groups. With this knowledge at hand, we managed to direct the chemical conversion of the oAP precursor to yield either solely bCDs or yCDs by adding a catalyst (either the Lewis acid AlCl3·6H2O or the Lewis base NaOH) that selectively and efficiently activated only one of the reaction pathways. This demonstration is important in that it shows that the synthesis of CDs with desired properties can be realized with efficient rational instead of trial-and-error means.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


