黄芩苷通过肠道微生物群介导的 cAMP/PKA/NF-κB 通路缓解溃疡性结肠炎
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-10-15
DOI:10.1016/j.bbrc.2024.150837
引用次数: 0
摘要
目的溃疡性结肠炎(UC)是一种慢性、非特异性结肠炎症,其特点是反复发作,且明显缺乏有效的药物治疗。黄芩苷是一种天然成分,具有明显的药理作用和治疗各种疾病的潜力。然而,人们对其对 UC 的作用还不完全了解,其确切的机制也有待破解。本研究旨在评估黄芩苷的疗效,并阐明其治疗 UC 的内在机制。方法本研究利用葡聚糖硫酸钠(DSS)诱导的小鼠来评估黄芩苷对 UC 的治疗潜力,并阐明涉及肠道微生物群的机制。研究还使用了抗生素鸡尾酒(ABX)和粪便微生物群移植(FMT)来确定肠道微生物群的作用机制。结果表明,黄芩苷在治疗 UC 方面有多种益处,包括减轻体重下降、减缓疾病进展和减少结肠结构的炎症损伤。服用黄芩苷后,肠道微生物群得到了改善,从而产生了这些效果。粪便代谢组显示,黄芩苷可选择性地缓解DSS诱导的肠道微生物群衍生代谢物失调,包括乙醇酸、γ-氨基丁酸、谷氨酸、色氨酸、黄嘌呤和β-羟基丙酮酸。网络药理学分析以及体内实验验证表明,cAMP/PKA/NF-κB 通路与这些代谢物治疗 UC 的作用有关,这可能是黄芩苷治疗 UC 的作用机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
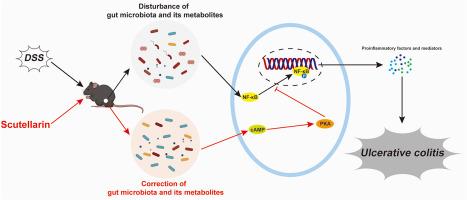
Scutellarin alleviated ulcerative colitis through gut microbiota-mediated cAMP/PKA/NF-κB pathway
Purpose
Ulcerative colitis (UC) is a chronic, non-specific inflammatory condition of the colon, characterized by recurrent episodes and a notable lack of effective pharmacological treatments. Scutellarin, a natural component, exhibits appreciable pharmacological effects and therapeutic potential for various diseases. However, its effects on UC are not fully understood, and the precise mechanisms remain to be deciphered. This study aimed to assess the therapeutic efficacy of scutellarin and elucidate its underlying mechanisms in treating UC.
Methods
This study utilized dextran sulfate sodium (DSS)-induced mice to evaluate the therapeutic potential of scutellarin against UC and to elucidate the mechanisms involving the gut microbiota. An antibiotics cocktail (ABX) and fecal microbiota transplantation (FMT) were also used to determine the mechanistic role of the gut microbiota. An integrative approach combining fecal metabolomics and network pharmacology analysis was used to explore the gut microbiota-directed molecular mechanism.
Results
The results showed that scutellarin provided various therapeutic benefits in UC management, including alleviating weight loss, slowing disease progression, and reducing inflammatory damage in colon structures. The improved gut microbiota after scutellarin administration contributed to these effects. Fecal metabolome revealed that scutellarin selectively mitigated DSS-induced dysregulation of gut microbiota-derived metabolites, including glycolic acid, γ-aminobutyric acid, glutamate, tryptophan, xanthine, and β-hydroxypyruvate. Network pharmacology analysis, along with in vivo experimental verification, implicated the cAMP/PKA/NF-κB pathway in the action of these metabolites in treating UC, which may be the mechanism responsible for scutellarin's curative effects on UC.
Conclusion
This study demonstrates the potential of scutellarin in alleviating UC by activating the cAMP/PKA/NF-κB pathway through gut microbiota-derived metabolites, highlighting scutellarin as a promising therapeutic agent for UC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


