表征支撑双金属铂锑团簇的表面成分:簇-支撑相互作用和表面吸附剂的影响
IF 1.8
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
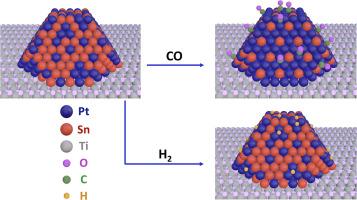
通过扫描隧道显微镜、低能离子散射 (LEIS)、X 射线光电子能谱和温度编程解吸 (TPD) 对 TiO2(110) 和高取向热解石墨 (HOPG) 表面上的铂锰双金属团簇进行了表征;还进行了密度泛函理论 (DFT) 计算,以更好地了解 CO 和 D2 在铂锰表面的吸附情况。在铂的覆盖率为 2 ML 和锡的覆盖率为 2 ML 的 TiO2 上,两种沉积阶次都能形成完全的双金属簇,因为第一种金属的簇完全覆盖了表面,这样第二种金属的所有原子都融入了现有的簇中。与此相反,在 HOPG 上,由于 HOPG 的高迁移率和微弱的簇支撑相互作用,形成了大得多的 2 ML 单金属簇(高 30 Å),这些簇并没有完全覆盖表面,而第二种金属的沉积既会产生较大的簇,也会产生较小的簇。尽管不同沉积顺序和支持物的簇形态各异,但 LEIS 实验表明,在所有情况下,PtSn 簇表面都富含 Sn,这是基于 Sn 的表面自由能低于 Pt 所预期的。此外,在有 Pt 存在的情况下,所有表面上观察到的 Sn(3d5/2) 结合能都发生了 +0.2 eV 的移动,这与 PtSn 合金的形成是一致的。在二氧化钛上沉积 2 ML Sn 会产生二维簇,在簇-支撑界面上 Sn 被氧化,二氧化钛被还原,但在 Sn 簇中添加铂会导致 Sn 从该界面扩散开,使 Sn 处于金属态。在 Sn 覆盖率不断增加的 2 ML Pt/TiO2 上进行的 TPD 实验表明,D2 的吸附位点数量在 0.5 ML 时急剧下降至近乎为零,而 CO 的吸附只有在 Sn 覆盖率高达 2 ML 时才降至零。对 Sn 修饰的铂表面和块体结构进行的 DFT 研究表明,在低 Sn 覆盖率(≤0.25 ML)条件下吸附 CO 时,强烈的 Pt-CO 相互作用会诱导铂向簇表面扩散并形成块体 Pt3Sn 合金,而吸附 D2 不会导致与铂表面产生足以诱导合金形成的相互作用。单个锡原子通过位阻和向铂提供电子密度阻止了 D2 在四个相邻铂原子上的吸附。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterizing the surface compositions of supported bimetallic PtSn clusters: Effects of cluster-support interactions and surface adsorbates
PtSn bimetallic clusters on TiO2(110) and highly oriented pyrolytic graphite (HOPG) surfaces have been characterized by scanning tunneling microscopy, low energy ion scattering (LEIS), X-ray photoelectron spectroscopy, and temperature programmed desorption (TPD); density functional theory (DFT) calculations have also been performed to better understand adsorption of CO and D2 on the PtSn surfaces. On TiO2 at coverages of 2 ML of Pt and 2 ML of Sn, exclusively bimetallic clusters are formed for both orders of deposition because clusters of the first metal completely cover the surface such that all atoms of the second metal are incorporated into the existing clusters. In contrast, on HOPG, the high mobility and weak cluster-support interactions on HOPG result in much larger 2 ML monometallic clusters (∼30 Å high) that do not completely cover the surface, and deposition of the second metal produces larger clusters as well as smaller ones. Despite the difference in cluster morphologies for the different orders of deposition and supports, the LEIS experiments demonstrate that in all cases, the PtSn clusters are rich in Sn at the surface, as expected based on the lower surface free energy for Sn compared to Pt. Furthermore, the +0.2 eV shift in the Sn(3d5/2) binding energy observed on all surfaces in the presence of Pt is consistent with PtSn alloy formation. Deposition of 2 ML of Sn on TiO2 produces two-dimensional clusters with oxidation of Sn and reduction of titania at the cluster-support interface, but addition of Pt to the Sn clusters causes Sn to diffuse away from this interface, leaving Sn in the metallic state. TPD experiments on 2 ML Pt/TiO2 with increasing coverages of Sn show that the number of adsorption sites for D2 sharply decreases to nearly zero at 0.5 ML, while CO adsorption decreases to zero only at much higher Sn coverages of 2 ML. DFT studies for Sn modified Pt surfaces and bulk structures demonstrate that for CO adsorption at low Sn coverages (≤0.25 ML), the strong Pt-CO interactions induce diffusion of Pt to the cluster surface and the formation of a bulk Pt3Sn alloy, whereas D2 adsorption does not lead to interactions with the Pt surface that are strong enough to induce alloy formation. A single Sn adatom prevents D2 adsorption on four neighboring Pt atoms via site-blocking and the donation of electron density to Pt.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


