分泌的载脂蛋白重塑了黑色素瘤细胞易受铁中毒影响的状态
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
黑色素瘤的一个主要治疗障碍是以不同代谢特征为标志的多种细胞状态并存。从增殖性黑色素瘤表型向侵袭性黑色素瘤表型的转变与铁中毒易感性的增加有关。然而,控制黑色素瘤细胞不同状态下铁蛋白沉积易感性的调控回路尚不清楚。在这项工作中,我们发现载脂蛋白 E(APOE)是黑色素瘤 MITFhigh/AXLlow 增殖/铁蛋白沉着病抗性与 MITFlow/AXLhigh 侵袭/铁蛋白沉着病敏感状态之间的首要脂质代谢基因。从机理上讲,MITFhigh/AXLlow 细胞分泌的载脂蛋白通过减少易发生过氧化反应的多不饱和脂肪酸的含量和提高体外及体内 GPX4 的水平,保护侵袭性表型免受铁变态反应诱导剂的影响。全外显子组测序表明,黑色素瘤患者的 APOE 高表达与对铁凋亡的抵抗有关,与 APOE 种系状态无关。总之,我们发现黑色素瘤细胞状态之间存在一种依赖于分泌型载脂蛋白的抗铁机制,APOE高表达是黑色素瘤对铁中毒反应差的潜在生物标志物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
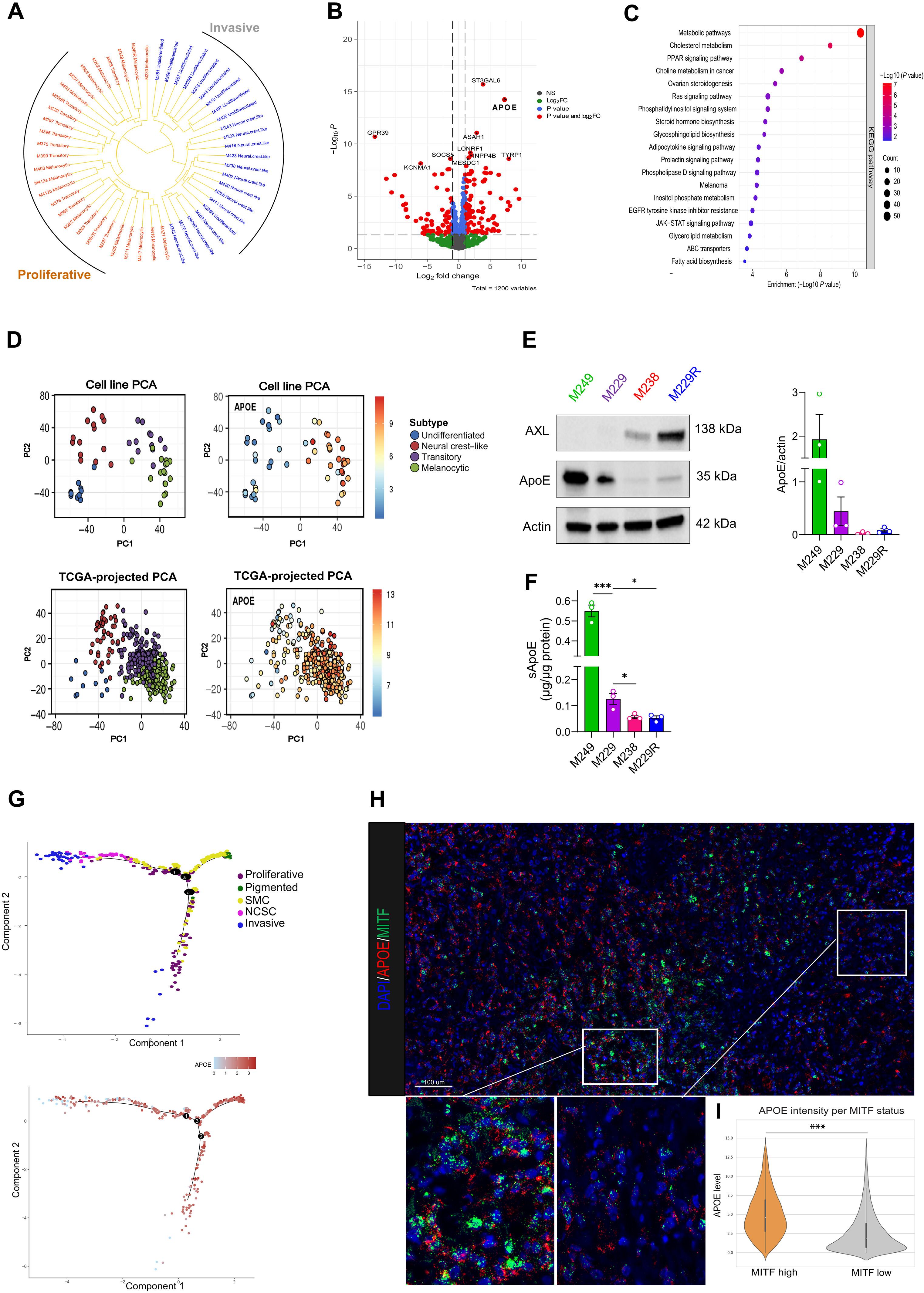
Secreted Apoe rewires melanoma cell state vulnerability to ferroptosis
A major therapeutic barrier in melanoma is the coexistence of diverse cellular states marked by distinct metabolic traits. Transitioning from a proliferative to an invasive melanoma phenotype is coupled with increased ferroptosis vulnerability. However, the regulatory circuits controlling ferroptosis susceptibility across melanoma cell states are unknown. In this work, we identified Apolipoprotein E (APOE) as the top lipid-metabolism gene segregating the melanoma MITFhigh/AXLlow proliferative/ferroptosis-resistant from MITFlow/AXLhigh invasive/ferroptosis-sensitive state. Mechanistically, ApoE secreted by the MITFhigh/AXLlow cells protects the invasive phenotype from ferroptosis-inducing agents by reducing the content of peroxidation-prone polyunsaturated fatty acids and boosting GPX4 levels both in vitro and in vivo. Whole-exome sequencing indicates that APOEhigh expression in patients with melanoma is associated with resistance to ferroptosis, regardless of APOE germline status. In aggregate, we found a ferroptosis-resistance mechanism between melanoma cell states relying on secreted ApoE and APOEhigh expression as a potential biomarker for poor ferroptosis response in melanoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


