血淋巴蛋白酶-17b能激活proHP6,从而刺激狐猴的黑化和Toll信号传导
IF 3.2
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
曼杜克蛇血淋巴蛋白酶-6(HP6)在协调丙酚氧化酶(PPO)激活和 Toll 信号转导等抗微生物反应中发挥着核心作用。我们之前的研究表明,HP5 和 GP6 分别激活幼虫血淋巴和胚外组织中的原 HP6。在此,我们报告了 HP17b 作为另一种 HP6 激活酶的特性及其在血淋巴中受多种丝蛋白调控的情况。在杆状病毒感染的Sf9细胞中表达的HP17b前体在两个位点自发裂解,这些产物被一起纯化为一种名为HP17b′的制剂,它是proHP17b、35 kDa中间体和HP17b的混合物。HP17b′ 将 proHP6 转化为 HP6。正如之前所报道的,HP6 可将 PPO 激活蛋白酶-1(PAP1)和 HP8 的前体转化为活性形式。HP8 可激活 proSpӓtzle-1 启动 Toll 信号转导。我们发现,HP17b′可直接激活原SPHI和II,形成PAP1激活PPO的辅助因子。在幼虫血淋巴中添加 HP17b′、HP17b 或 proHP17b 能显著增加 PPO 的活化。在含有HP17b′、HP17b或proHP17b的反应中,加入黄体小球菌并不能增强PPO的活化。使用 HP17b 抗体,我们从诱导血浆中分离出了 HP17b 片段和相关蛋白(如 serpin-4)。Serpin-1A、1J、1J′、4、5或6通过与活性HP17b形成共价复合物,减少了HP17b对proHP6的激活。我们在条纹期噬蛹的血淋巴中检测到了原 HP17b 的裂解活性,但由于其高度不稳定性,我们未能纯化这种蛋白酶。其他已知的 HPs 在体外没有激活 proHP17b。这些结果表明,HP17b是一种由未知内肽酶激活的剪切域蛋白酶,可对危险信号做出反应,并受多种丝蛋白调节。本文章由计算机程序翻译,如有差异,请以英文原文为准。
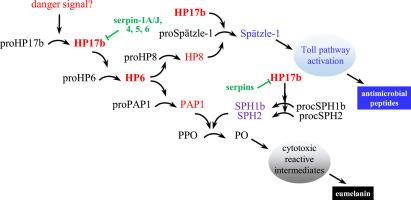
Hemolymph protease-17b activates proHP6 to stimulate melanization and Toll signaling in Manduca sexta
Manduca sexta hemolymph protease-6 (HP6) plays a central role in coordinating antimicrobial responses, such as prophenoloxidase (PPO) activation and Toll signaling. Our previous studies indicated that HP5 and GP6 activate proHP6 in larval hemolymph and extraembryonic tissues, respectively. Here, we report the characterization of HP17b as another HP6 activating enzyme and its regulation by multiple serpins in hemolymph. The precursor of HP17b expressed in baculovirus infected Sf9 cells became spontaneously cleaved at two sites, and these products were purified together in one preparation named HP17b′, a mixture of proHP17b, a 35 kDa intermediate, and HP17b. HP17b′ converted proHP6 to HP6. As reported before, HP6 converted precursors of PPO activating protease-1 (PAP1) and HP8 to their active forms. HP8 activates proSpӓtzle-1 to turn on Toll signaling. We found HP17b′ directly activated proSPHI and II to form a cofactor for PPO activation by PAP1. Supplementation of larval hemolymph with HP17b′, HP17b, or proHP17b significantly increased PPO activation. Adding Micrococcus luteus to the reactions did not enhance PPO activation in the reactions containing HP17b′, HP17b, or proHP17b. Using HP17b antibodies, we isolated from induced plasma HP17b fragments and associated proteins (e.g., serpin-4). Serpin-1A, 1J, 1J′, 4, 5, or 6 reduced the activation of proHP6 by HP17b’ through formation of covalent complexes with active HP17b. We detected an activity for proHP17b cleavage in hemolymph from bar-stage pharate pupae but failed to purify the protease due to its high instability. Other known HPs did not activate proHP17b in vitro. Together, these results suggest that HP17b is a clip-domain protease activated by an unknown endopeptidase in response to a danger signal and regulated by multiple serpins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


