利用 RSM 和 ANN 建模方法优化 LDH/精氨酸复合珠对磷酸盐离子的去除效果
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本研究开发了层状双氢氧化物(MgAl)和海藻酸复合珠(LDH/海藻酸),并将其用作去除磷酸盐离子的低成本环保型吸附剂。扫描电镜分析证实,海藻酸钠成功地融入了 LDH 结构中。干燥后的珠子表面明显粗糙,这促进了分子运动,并有可能增强对污染物的吸附。复合材料零电荷点的 pH 值(pHPZC)为 7.41。这一结果表明,当 pH 值低于 pHPZC 时,负离子倾向于通过静电相互作用力在吸附剂表面吸附正电荷。另一方面,pH 值高于 pHPZC 的表面大多带有负电荷。为了评估和比较响应面方法(RSM)和人工神经网络方法(ANN)对吸附过程的预测能力,实验同时改变了三个因素。两种方法都显示出了准确预测吸附过程的强大能力。然而,与人工神经网络方法相比,响应面方法的预测误差更小。采用响应面方法中的中央复合设计(CCD-RSM)来优化吸附过程的实验条件。该模型考虑了三个因素:吸附剂剂量 (A)、初始浓度 (B) 和接触时间 (C)。其中,二次项(B2)对磷酸盐离子吸附率的影响最大。分析得出的 R2 值为 0.94,表明数据拟合得非常好。研究结果表明,增加接触时间和降低初始浓度可以提高磷酸盐离子的去除效率,而吸附剂剂量几乎没有影响。人工神经网络(ANN)模型有效地预测了 LDH/海藻酸盐复合珠对磷酸盐离子的吸附修复效果,模型输出结果与实验数据之间的判定系数(R2 = 0.984)很高。该研究强调了 LDH/海藻酸复合珠作为天然吸附剂去除水溶液中磷酸盐离子的巨大潜力。这些结果凸显了用于磷酸盐吸附的吸附剂的环保性和高效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
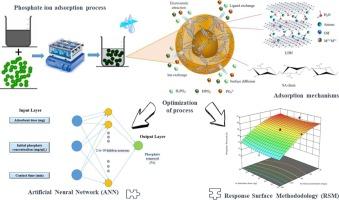
Exploiting RSM and ANN modeling methods to optimize phosphate ions removal using LDH/alginate composite beads
In the present work, layered double hydroxide (MgAl) and alginate composite beads (LDH/alginate) were developed and used as low-cost and environmentally friendly adsorbent for phosphate ions removal. The successful incorporation of sodium alginate into the LDH structure was confirmed through SEM analysis. The dried beads exhibited a notably rough surface, which promotes molecular movement and potentially enhances pollutant adsorption. The pH of zero charge point (pHPZC) of the composite was found to be 7.41. This result implies that negative ions have a tendency to draw positive charges on the adsorbent surface via electrostatic interaction forces when the pH is lower than the pHPZC. On the other hand, a surface that has a pH higher than pHPZC mostly has a negative charge. The percentage removal and adsorption capacity were investigated as a function of contact time.
Experiments were carried out by simultaneously varying three factors, in order to evaluate and compare the predictive capabilities of the response surface methodology (RSM) and the artificial neural network approach (ANN) for the adsorption process. Both methods demonstrated a strong ability to accurately predict the adsorption process. However, the response surface methodology exhibited a lower prediction error compared to the artificial neural network approach.
The central composite design within response surface methodology (CCD-RSM) was employed to optimize the experimental conditions for the adsorption process. The model, which considers three factors: adsorbent dose (A), initial concentration (B), and contact time (C), proved to be significant. Among these, the quadratic term (B2) had the most substantial impact on the phosphate ion adsorption rate. The analysis yielded an R2 value of 0.94, indicating an excellent fit to the data. The findings suggest that increasing contact time and reducing the initial concentration improve phosphate ion removal efficiency, while the adsorbent dose has little to no effect. The Artificial Neural Network (ANN) model effectively predicted the adsorptive remediation of phosphate ions onto LDH/alginate composite beads, achieving a high coefficient of determination (R2 = 0.984) between the model outputs and the experimental data. The study highlights the significant potential of LDH/alginate composite beads as a natural adsorbent for the removal of phosphate ions from aqueous solutions. These results underscore the environmental friendliness and efficiency of the adsorbent used for phosphate adsorption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


