清心解郁颗粒通过激活 ANXA1/FPR2 轴抑制中性粒细胞胞外捕获物缓解心肌梗死
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景心肌梗塞(MI)是冠状动脉疾病(CAD)最严重的表现形式,是心血管疾病防治的首要问题。临床证据表明,清心解郁颗粒(QXJYG)对治疗心肌梗死患者有显著疗效。材料和方法通过结扎左前降支(LAD)动脉建立大鼠心肌梗死模型。通过超声心动图评估 QXJYG 对心肌梗死大鼠心功能损伤的影响,通过苏木精-伊红(H&E)染色和 Masson 染色评估 QXJYG 治疗后心肌细胞形态和心肌纤维化的改善情况。采用 UPLC-Q-TOF/MS 技术鉴定了血液中 QXJYG 的化学成分。此外,还根据疾病基因-药物靶点网络分析,推测了琼XJYG对心肌缺血的治疗作用的分子机制。结果 心肌梗死后大鼠心功能明显受损,表现为梗死面积扩大、心肌细胞排列紊乱、心肌纤维化加重。QXJYG能明显增强心肌梗死大鼠的心功能,减轻心肌组织的病理损伤。通过网络药理学分析,我们发现FPR2可能是QXJYG发挥心脏保护作用的潜在靶点。QXJYG 能明显降低心肌梗死大鼠中性粒细胞胞外捕获物(NET)的水平,具体表现为组蛋白 DNA 水平以及髓过氧化物酶(MPO)和瓜氨酸组蛋白 H3(CitH3)蛋白的表达显著降低。此外,QXJYG 还能上调 MI 大鼠体内 ANXA1 和 FPR2 蛋白的水平。给予 FPR2 的特异性抑制剂 WRW4 后,MI 大鼠体内的 FPR2 水平明显降低,这与 MI 损伤加重和 NET 水平升高有关。当 WRW4 与 QXJYG 合用时,QXJYG 对 MI 的心脏保护作用明显减弱。结论 QXJYG 通过激活 ANXA1/FPR2 轴来抑制 NETs,从而减轻心肌梗死大鼠的心脏组织损伤。这些研究结果拓展了我们对 QXJYG 治疗效果的认识,为 QXJYG 的临床应用提供了科学依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
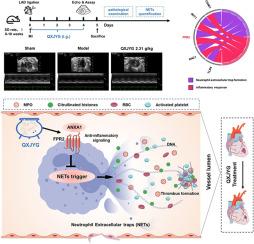
Qingxin Jieyu Granule alleviates myocardial infarction through inhibiting neutrophil extracellular traps via activating ANXA1/FPR2 axis
Background
Myocardial infarction (MI), representing the most severe manifestation of coronary artery disease (CAD), stands as a primary concern in the prevention and management of cardiovascular diseases. Clinical evidence demonstrates that Qingxin Jieyu Granule (QXJYG) is efficacious in treatment of MI patients. However, the mechanisms underlying its therapeutic effects remain to be elucidated.
Purpose
This study aimed to evaluate the effects of QXJYG on MI and investigate its underlying mechanisms.
Materials and methods
The MI model in rats was developed through ligating the left anterior descending (LAD) artery. The effect of QXJYG on cardiac function impairment in MI rats was assessed by echocardiography, while the improvement of cardiomyocyte morphology and myocardial fibrosis after treatment with QXJYG was evaluated through hematoxylin-eosin (H&E) staining and Masson staining. The chemical constituents of QXJYG in blood were identified by using the UPLC-Q-TOF/MS technique. Furthermore, the molecular mechanism underlying the QXJYG therapeutic effect in MI was postulated based on the disease gene-drug target network analysis. Other technical methods such as ELISA, immunohistochemical staining, Western Blot analysis and application of pharmacological inhibitors were employed to verify the effectiveness of QXJYG in treating MI and explore its potential molecular targets.
Results
The cardiac function in experimental rats post-MI was significantly impaired, as evidenced by an enlarged infarction area, disordered arrangement of cardiomyocytes, and aggravated myocardial fibrosis. QXJYG treatment significantly enhanced the cardiac function and reduced the pathological damage of the cardiac tissue in MI rats. Through the network pharmacology analysis, we identified that FPR2 might be a potential target of QXJYG in its cardiac protection role. QXJYG markedly downregulated the level of neutrophil extracellular traps (NETs) in MI rats, specifically manifested as a significant reduction in the Histone-DNA level and expression of myeloperoxidase (MPO) and citrullinated histone H3 (CitH3) proteins. Furthermore, QXJYG upregulated the levels of ANXA1 and FPR2 proteins in MI rats. The level of FPR2 was markedly reduced in MI rats upon administration of WRW4, a specific inhibitor of FPR2, which was associated with exacerbated MI injury and an elevated level of NETs. When WRW4 was co-administered with QXJYG, the cardioprotective effects of QXJYG on MI were significantly diminished. However, the addition of DNase I did not result in significant changes of the outcomes in MI rats after QXJYG intervention.
Conclusion
QXJYG treatment alleviates cardiac tissue injury in MI rats by inhibiting NETs through activating the ANXA1/FPR2 axis. The findings extend our understanding of the therapeutic effectiveness of QXJYG and offer a scientific foundation for the clinical utilization of QXJYG.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


