掺氮 TIO2/g-C3N4 纳米催化剂用于亚甲基蓝染料的光降解
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
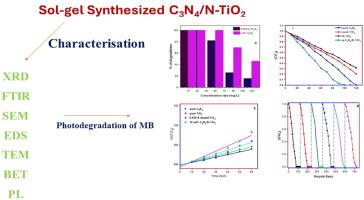
本研究采用乙醇分散法制备 g-C3N4/N-TiO2 纳米催化剂结构。利用溶胶-凝胶合成法通过尿素热分解合成 N-TiO2 和 g-C3N4。利用聚光成像、紫外可见 DRS、XRD、傅立叶变换红外光谱、扫描电镜、BET 和 TEM 揭示了所制备光催化剂的物理化学性质。g-C3N4 和 N-TiO2 掺杂形成的纳米复合结构提高了光催化活性,影响了电荷分离性能和使用寿命。该系统还具有收集可见光和紫外线的能力。即使在连续四次循环后,甲基溴的效率仍高于 85%。捕获实验表明,甲基溴的分解主要是由 h+ 和 O22- 自由基引起的。动力学研究结果表明,g-CN4/N-TiO2 纳米复合材料的降解速率超过了 N-TiO2 纳米粒子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nitrogen doped TIO2/g-C3N4 nanocatalyst for photodegradation of methylene blue dye
In this study, the ethanol dispersion procedure was implemented to prepare the g-C3N4/N–TiO2 nanocatalyst structure. Sol-gel synthesis is utilized for synthesizing N–TiO2 and g-C3N4 via urea thermal decomposition. Physicochemical properties of produced photocatalysts were revealed using PL imaging, UV–vis DRS, XRD, FTIR, SEM, BET, and TEM. Evaluation of photocatalytic activity of synthetic photocatalysts by exposure to sunlight and degradation of methylene blue (MB) dye. g-C3N4/N–TiO2 NCs showed excellent photocatalytic activity, reaching 96 % MB degradation efficiency in 80 min. g-C3N4 and N–TiO2 doped form a nanocomposite structure that increases photocatalytic activity, affecting charge separation performance and service life. The system also exhibits the ability to collect visible and ultraviolet light. Even after four consecutive cycles, the efficiency of the MB is still above 85 %. Capture experiments showed that the decomposition of MB was mainly caused by h+ and O22− radicals. The results of kinetic investigations indicate that the degradation rate of g-C3N4/N–TiO2 nanocomposites surpasses that of N–TiO2 nanoparticles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


