膳食二氧化钛纳米颗粒通过扰乱肠干细胞的功能损害肠上皮再生
IF 3.9
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
肠道健康与肠道干细胞(ISC)密切相关,肠道干细胞对肠腔中的有害物质高度敏感。然而,人们对食品添加剂对肠干细胞的影响了解有限。本研究旨在探讨膳食中的二氧化钛纳米颗粒(TiO2 NPs)与二氧化钛微颗粒(TiO2 MPs)相比,在应对右旋糖酐硫酸钠(DSS)诱导的小鼠肠炎时,对与ISC相关的肠道健康的影响以及相关机制。我们发现,暴露于1%(w/w)TiO2 NPs会加重DSS诱导的小鼠肠炎,而暴露于TiO2 MPs则观察不到这种效应。此外,在DSS诱导的肠炎中暴露于1%(w/w)TiO2 NPs会使ISC介导的肠上皮再生恶化,因为它降低了上皮细胞的增殖和上皮细胞的周转率,同时增加了上皮细胞的死亡。同时,我们利用三维肠道类器官模型发现,20 μg/mL TiO2 NPs会损害ISC的功能,并破坏ISC在体内外的命运分化。此外,TiO2 NPs还阻碍了β-catenin的核转位,降低了Wnt信号的整体输出。总之,TiO2 NPs通过一种涉及Wnt信号转导的机制,扰乱了ISC的功能和命运分化,从而恶化了DSS诱导的肠炎小鼠的肠上皮再生。这些发现凸显了膳食二氧化钛纳米粒子对ISC的不利影响,并为二氧化钛食品添加剂的粒度优化提供了启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
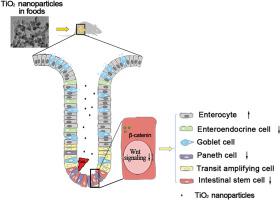
Dietary titanium dioxide nanoparticles impair intestinal epithelial regeneration by perturbating the function of intestinal stem cells
Intestinal health is closely linked to intestinal stem cells (ISCs), which are highly sensitive to the harmful substances in the lumen. However, there is limited knowledge regarding the effects of food additives on ISCs. This study aims to investigate the impact of dietary titanium dioxide nanoparticles (TiO2 NPs) compared with titanium dioxide microparticles (TiO2 MPs) on intestinal health associated with ISCs in response to dextran sodium sulfate (DSS)-induced enteritis in mice, as well as the related mechanism. We found that exposure to 1% (w/w) TiO2 NPs aggravated DSS-induced enteritis in mice, while this effect could not be observed under exposure to TiO2 MPs. Additionally, 1% (w/w) TiO2 NPs exposure under DSS-induced enteritis worsened the ISC-mediated regeneration of intestinal epithelium by decreasing the epithelial cell proliferation and epithelial turnover rate while increasing epithelial cell death. Meanwhile, using a 3D intestinal organoid model, we discovered that 20 μg/mL TiO2 NPs impaired ISC function and disrupted ISC fate specification both ex vivo and in vitro. Furthermore, TiO2 NPs hindered the nuclear translocation of β-catenin, reducing the overall output of Wnt signaling. Together, TiO2 NPs deteriorated the intestinal epithelial regeneration of mice with DSS-induced enteritis by perturbating ISC function and fate specification through a mechanism involving Wnt signaling. These findings highlight the adverse effect of dietary TiO2 NPs on ISCs and shed light on the particle size optimization of TiO2 food additive.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


