开发和评估用于有效消除水溶液中有害农药的钒基金属有机框架:吸收机制、吸附能力、吸收率以及通过 Box-Behnken 设计提高吸收率
IF 5.8
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
钒金属有机框架(V-MOF)在高效去除来自农业废水甲基对硫磷(MP)农药的水中污染物方面具有巨大潜力。利用 SEM、FT-IR、XPS)、XRD 和 BET 分析等多种方法,成功合成并表征了该吸附剂。测量到的孔隙尺寸为 1.33 nm,符合 IUPAC 系统中的微孔分类。吸附前,材料的表面积为 1489.42 m2/g,孔体积为 0.98 cc/g。吸附 MP 后,表面积、孔径和孔体积分别降至 1268.42 m2/g、1.12 nm 和 0.64 cc/g。材料物理性质的变化表明吸附过程产生了影响。7.2 是通过表面表征控制零电荷点的结果。这一信息表明,当 pH 值低于 7.2 时,吸附剂表面带有正电荷,而当 pH 值高于该值时,表面则带有负电荷。我们还研究了 pH 值对吸附平衡的影响。虽然与 Langmuir 等温拟合,但 MP 在 V-MOF 上的吸附动力学却是伪二阶拟合。由于吸附能为 23.62 kJ.mol-1,因此化学吸附很可能是吸附模式。热力学参数研究得出的焓(ΔHo)值为正值,表明在该温度范围内,农药吸附过程是内热的,为 32.79 kJ.mol-1。熵(ΔSo)读数为正,表明在吸附过程中系统的随机性增加,达到 119 J.mol-1K-1,而且随着温度的升高,ΔGo 的负值也在增加。通过在实验室环境中过滤废水样本,对所推荐的吸附剂的效果进行了评估。根据假设,V-MOF 和 MP 将通过孔隙填充、π-π 相互作用、H 键、静电接触和其他可能的方法发生相互作用。详细考虑这种相互作用的具体细节,对于理解吸附的本质和有效构建用于实际应用的吸附剂至关重要。通过使用 V-MOF 吸附技术,水过滤和工业废水处理变得简单而有效。结果表明,pH=6 时的最大吸附容量为 383.6 毫克/克。为了评估吸附剂的再生能力,还进行了更多的测试,结果表明,即使经过六个以上的循环,吸附剂的再生能力仍在持续。使用 XRD 和 FT-IR 证实了吸附剂在再生过程中的稳定性。然后采用盒式贝肯设计(BBD)来优化吸附结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
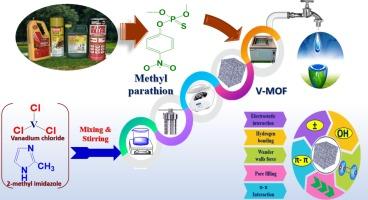
Development and assessment of vanadium-based metal–organic frameworks for the effective elimination of hazardous pesticides from aqueous solutions: Mechanism of uptake, adsorption capacities, rate of uptake, and enhancement via the Box-Behnken design
Vanadium metal–organic frameworks (V-MOF) has a great potential to remove contaminants from water that come from agriculture wastewater methyl parathion (MP) pesticides with high effectiveness. Using a variety of methods, such as SEM, FT-IR, XPS), XRD, and BET analysis, the adsorbent was successfully synthesized and characterized. The dimensions of the pores measured at 1.33 nm correspond to the classification of micropores under the IUPAC system. Before the adsorption process, the material had a surface area of 1489.42 m2/g and a pore volume of 0.98 cc/g. After MP adsorption, the surface area, pore size, and pore volume decreased to 1268.42 m2/g, 1.12 nm, and 0.64 cc/g, respectively. Changes in the material’s physical properties indicate that the adsorption process had an effect. 7.2 was the result of controlling the point of zero charge through surface characterization. This information suggests that the surface of the adsorbent has a positive charge at pH below 7.2 and at the pH higher than this value the surface will have a negative charge. It was also looked into how pH affected the adsorption equilibrium. Although fitting to Langmuir isothermally, the kinetics of MP adsorption onto V-MOF are pseudo-second-order fitting. It is very probable that chemisorption was the mode of adsorption because the adsorption energy was 23.62 kJ.mol−1. The enthalpy (ΔHo) values obtained as a result of studying the thermodynamic parameters are positive, demonstrating that, within this temperature range, the pesticide adsorption process was endothermic., measuring 32.79 kJ.mol−1. Entropy (ΔSo) readings that are positive indicate that the system’s randomness increased during the adsorption process, reaching 119 J.mol−1K−1, and with rising temperatures, the negative of ΔGo rise. The effectiveness of the recommended adsorbent was evaluated by filtering wastewater samples in a laboratory environment. It is hypothesized that V-MOF and MP will interact by pore filling, π-π interaction, H-bonding, electrostatic contact, and other possible methods. Considering the specifics of this interaction in great detail is essential to comprehending the nature of adsorption and effectively constructing the adsorbent for use in real-world applications. Water filtering and the treatment of industrial effluents were made simple and effective by the use of V-MOF adsorbent technology. The results indicated that 383.6 mg/g was the maximal adsorption capacity at pH = 6. To evaluate the renewal of the adsorbent, more tests were carried out, and the outcomes showed that the renewal continued even after more than six cycles. The stability of the adsorbent during regeneration was confirmed by using XRD and FT-IR. The Box Behnken design (BBD) was then employed to optimize the adsorption outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Saudi Chemical Society
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
8.90
自引率
1.80%
发文量
120
审稿时长
38 days
期刊介绍:
Journal of Saudi Chemical Society is an English language, peer-reviewed scholarly publication in the area of chemistry. Journal of Saudi Chemical Society publishes original papers, reviews and short reports on, but not limited to:
•Inorganic chemistry
•Physical chemistry
•Organic chemistry
•Analytical chemistry
Journal of Saudi Chemical Society is the official publication of the Saudi Chemical Society and is published by King Saud University in collaboration with Elsevier and is edited by an international group of eminent researchers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


