气体扩散电极通过 O2 电还原生成 H2O2 的综述:从均相电催化到异相电催化
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
近年来,电化学研究的重点是开发一种通过双电子氧还原反应(2eORR)原位生成过氧化氢(H2O2)的高效替代技术。本综述总结了电化学合成 H2O2 的最新进展,强调了气体扩散电极 (GDE) 的关键作用。首先,介绍了新型改性 GDE 的不同结构和组装工艺。然后总结了气体扩散电极的机理、制造方法和代表作品。此外,还分析了所采用的各种催化剂层(CL),旨在提高 2eORR 工艺的活性和选择性。随后,综述深入探讨了影响 H2O2 电化学生成的操作参数,包括电流/电位、气体流速、电解质类型、电解质 pH 值和温度等关键参数。这些参数会影响 O2 电还原的热力学和动力学,从而调节 H2O2 的产量。最后,我们探讨了通过 GDE 通过 2eORR 生产 H2O2 所面临的挑战和机遇。本文章由计算机程序翻译,如有差异,请以英文原文为准。
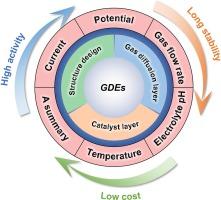
Review of H2O2 generation from O2 electroreduction by gas diffusion electrodes: From homogeneous to heterogeneous electrocatalysis
In recent years, electrochemical research has focused on developing an efficient alternative technology for the in-situ generation of hydrogen peroxide (H2O2) via the two-electron oxygen reduction reaction (2eORR). This comprehensive review summarizes the most recent advancements in the electrochemical synthesis of H2O2, emphasizing the pivotal role of gas diffusion electrodes (GDEs). First, different structures and assembly processes of novel modified GDEs were introduced. The mechanism, fabrication, and representative works of the GDE, were then summarized. Furthermore, the variety of catalyst layers (CL) employed were analyzed, aiming to enhance both the activity and selectivity of the 2eORR process. Subsequently, the review delves into the operational parameters that govern the electrochemical generation of H2O2, including critical parameters such as current/potential, gas flow rate, electrolyte type, electrolyte pH, and temperature. These parameters affect the thermodynamics and kinetics of O2 electroreduction, thereby modulating the yield of H2O2. Concludingly, the challenges and opportunities in H2O2 production through the 2eORR via GDEs were explored.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


