制备用作超级电容器电极的废轮胎/二氧化锰/聚苯胺三元纳米复合夹层结构多孔碳
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
开发包含多种电容材料的多组分电极已成为设计高性能超级电容器的可行策略。本文通过苯胺的原位化学氧化聚合合成了由聚苯胺(PANI)、二氧化锰(MnO2)和废轮胎多孔碳(PCDWT)组成的三明治结构三元复合材料。这种复合材料的特点是在绒球状 PCDWT/MnO2 表面紧密而均匀地包覆了蠕虫状 PANI,MnO2 在聚合过程中起到了反应模板的作用。三元纳米复合材料 PCDWT/MnO2/PANI-3 g 表现出优异的电化学性能,在三电极配置下,1.0 A/g 时的比电容达到 369.6F/g。值得注意的是,在 10 A/g 条件下进行 6,000 次充放电循环后,它仍能保持 95.5% 的初始电容。此外,用 PCDWT/MnO2/PANI-3 g 和 PCDWT 电极制造的非对称超级电容器在 0.5 A/g 时显示出 91.34F/g 的比电容。该装置在功率密度为 424.99 W kg-1 时可提供 36.66 Wh kg-1 的最大能量密度,在 10 A/g 条件下循环 10,000 次后,电容保持率为 87.72%。这种优异的电化学性能可归功于 PCDWT/MnO2 支架和 PANI 外层的协同效应,前者可增强电荷转移和电子传输,后者可提高 MnO2 的导电性、防止溶解并增加电活性位点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
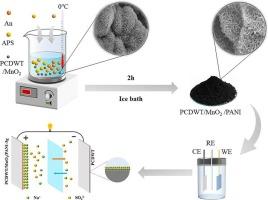
Preparation of sandwich-structured ternary nanocomposites porous carbon-derived from waste tires/manganese dioxide/polyaniline as electrode for supercapacitor
The development of multicomponent electrodes incorporating diverse capacitive materials has become a viable strategy for engineering high-performance supercapacitors. In this paper, a sandwich-structured ternary composite consisting of polyaniline (PANI), manganese dioxide (MnO2), and porous carbon-derived from waste tires (PCDWT) has been synthesized via in-situ chemical oxidative polymerization of aniline. The composite features worm-like PANI tightly and uniformly coated on pompon-like PCDWT/MnO2 surfaces, facilitated by MnO2 serving as a reactive template during polymerization. The ternary nanocomposite PCDWT/MnO2/PANI-3 g exhibited exceptional electrochemical performance, achieving a specific capacitance of 369.6F/g at 1.0 A/g in three-electrode configuration. Remarkably, it retained 95.5 % of its initial capacitance after 6,000 charge–discharge cycles at 10 A/g. Moreover, an asymmetric supercapacitor fabricated with PCDWT/MnO2/PANI-3 g and PCDWT electrodes exhibited a specific capacitance of 91.34F/g at 0.5 A/g. The device delivered a maximum energy density of 36.66 Wh kg−1 at a power density of 424.99 W kg−1 and maintained capacitance holdings of 87.72 % after 10,000 cycles at 10 A/g. This superior electrochemical performance can be attributed to the synergistic effects of the PCDWT/MnO2 scaffold, which enhances charge transfer and electron transport, and the outer PANI layer, which improves the electrical conductivity of MnO2, protects against dissolution, and increases electroactive sites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


