Alda-1 通过抑制 c-Jun N-terminal Kinase-2 活性的新机制,减轻酗酒导致的房性心律失常
IF 4.9
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
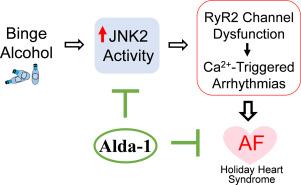
假日心脏综合征(HHS)是由过度酗酒引起的,而心房颤动(AF)是假日心脏综合征患者中最常见的心律失常。房颤与严重的发病率和死亡率相关,因此其预防和治疗在临床上备受关注。本研究确定了 Alda-1(一种成熟的心脏保护剂)的抗房颤作用,以及在我们表征良好的高血压和细胞模型中的基本作用机制。我们发现,Alda-1 能有效消除暴饮暴食酒精诱发的 Ca2+ 触发活动(Ca2+ 波、延长的 Ca2+ 瞬时舒张衰减)以及完整小鼠心房的心律失常诱导性。然后,我们证明酒精会损害人类 RyR2 通道(从器官捐献者的心脏中分离)。酒精导致的 RyR2 通道功能障碍在 Ca2+ 触发的心律失常活动中的功能作用在一个独特的功能缺失突变(RyR2E4872Q+/-)转基因小鼠模型中得到了证实。众所周知,Alda-1 能激活醛脱氢酶 2(ALDH2),这是酒精解毒过程中的一种关键酶。然而,我们发现在暴饮暴食酒精暴露的心脏和 H9c2 分化的心肌细胞中,ALDH2 的水平升高,而促凋亡与抗凋亡信号的平衡保持正常,这表明酒精-ALDH2-凋亡之间的联系不太可能是导致暴饮暴食酒精诱发心律失常的关键因素。我们以前曾报道过,暴饮暴食酒精激活的应激反应激酶 JNK2 是 Ca2+ 触发心房致心律失常的诱因。在这里,我们发现在离体的人类 RyR2 通道或完整的小鼠心房中抑制 JNK2 特异性可消除酒精诱发的 RyR2 通道功能障碍和 Ca2+ 触发的心律失常活动,这表明酒精-JNK2-RyR2 在心房致心律失常中具有很强的相互作用。此外,我们首次发现 Alda-1 可独立于 ALDH2 而抑制 JNK2(而非 JNK1)酶的活性,这反过来又减轻了酗酒诱发的 Ca2+ 触发的心房致心律失常。我们的研究结果为 Alda-1 的抗心律失常作用提供了新的机理认识,并表明 Alda-1 是一种潜在的预防性药物,可用于 HHS 患者的房颤治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Alda-1 attenuation of binge alcohol-caused atrial arrhythmias through a novel mechanism of suppressed c-Jun N-terminal Kinase-2 activity
Holiday Heart Syndrome (HHS) is caused by excessive binge alcohol consumption, and atrial fibrillation (AF) is the most common arrhythmia among HHS patients. AF is associated with substantial morbidity and mortality, making its prevention and treatment of high clinical interest. This study defines the anti-AF action of Alda-1 (an established cardioprotective agent) and the underlying mechanisms of the action in our well-characterized HHS and cellular models. We found that Alda-1 effectively eliminated binge alcohol-evoked Ca2+ triggered activities (Ca2+ waves, prolonged Ca2+ transient diastolic decay) and arrhythmia inducibility in intact mouse atria. We then demonstrated that alcohol impaired human RyR2 channels (isolated from organ donors' hearts). The functional role of alcohol-caused RyR2 channel dysfunction in Ca2+ triggered arrhythmic activities was evidenced in a unique transgenic mouse model with a loss-of-function mutation (RyR2E4872Q+/−). Alda-1 is known to activate aldehyde dehydrogenase 2 (ALDH2), a key enzyme in alcohol detoxification. However, we found an increased level of ALDH2 and a preserved normal balance of pro- vs anti-apoptotic signaling in binge alcohol exposed hearts and H9c2 differentiated myocytes, which suggests that the link of alcohol-ALDH2-apoptosis is unlikely to be a key factor leading to binge alcohol-evoked arrhythmogenicity. We have previously reported that binge alcohol-activated stress response kinase JNK2 causatively drives Ca2+-triggered atrial arrhythmogenicity. Here, we found that JNK2-specific inhibition in either isolated human RyR2 channels or intact mouse atria abolished alcohol-evoked RyR2 channel dysfunction and Ca2+ triggered arrhythmic activities, suggesting a strong alcohol-JNK2-RyR2 interaction in atrial arrhythmogenicity. Furthermore, we revealed, for the first time, that Alda-1 suppresses JNK2 (but not JNK1) enzyme activity independently of ALDH2, which in turn alleviates binge alcohol-evoked Ca2+ triggered atrial arrhythmogenesis. Our findings provide novel mechanistic insights into the anti-arrhythmic action of Alda-1 and suggest that Alda-1 represents a potential preventative agent for AF management for HHS patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


