印度东北部一些野生肉豆蔻的油脂化学潜力
IF 1.4
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
精油和固定油的工业用途非常广泛,包括香精香料、化妆品、保健身体护理、生物燃料和生物能源。肉豆蔻属植物传统上用于制作肥皂和蜡烛。研究的重点是提取阿萨姆邦及邻近地区肉豆蔻科植物的精油和固定油,并确定其特征。该研究首次从印度东北部的 Horsfieldia kingii 和 H. amygdalina 植物部分中发现了精油。GC-MS 分析显示,在 Horsfieldia kingii、H. amygdalina 和 Knema angustifolia 中发现了近 47 种化合物,后两者的成分相似。鉴定出的常见重要化合物约有 11 种,分别是 copaene(1.34-22.22%)、β-caryophyllene(0.34-4.36%)、caryophyllene oxide(0.82-50.43%)、humulene epoxide II(1.21-5.67%), δ-cadinol (1.10-7.92%), epi-γ-eudesmol (3.03-10.83%), globulol (0.82-42.28%), viridiflorol (2.34-39.14%), β-elemene (0.47-18.62%), shyobunol (1.37-7.5%) 和 t-cadinol (1.29-4.46%)。挥发物覆盖率较高的是杜松樟脑(70.85%)、氧化香叶烯(50.43%)、球醇(42.28%)、紫罗兰酚(39.14%)、α-蒎烯(36.56%)和β-硒烯(20.53%)。H.amygdalina、H.kingii、K.angustifolia、K.linifolia 和 K. tenuinervia 的核仁和肉豆蔻固定油的理化参数首次显示出 6.10-44.35%,其中肉豆蔻酸甲酯(20.87%-86.1%)、月桂酸甲酯(35.81%-40.02%)、油酸甲酯(30.2%-47.45%)、棕榈酸甲酯(19.16%-37.9%)含量丰富,大部分为饱和脂肪酸。野生肉豆蔻的精油和固定油都显示了野生生物废弃物作为新型可再生生物标志物来源的商业潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
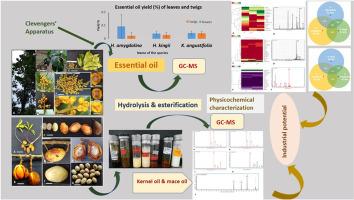
The oleochemical potential of some wild nutmegs from North East India
The essential and fixed oils cover a vast arena of industrial usage, including flavour-fragrances, cosmetics, health-body care, biofuel and bioenergy. The species of Myristicaceae were traditionally used to prepare soaps and candles. The research focused on extracting and characterising the essential and fixed oils of Myristicaceae from Assam and adjoining region. The study found essential oil for the first time from vegetative parts of Horsfieldia kingii and H. amygdalina from NE India. GC-MS analysis exhibited nearly 47 compounds among Horsfieldia kingii, H. amygdalina and Knema angustifolia, having compositional similarity among the latter two. About 11 common and significant compounds identified were copaene (1.34–22.22%), β-caryophyllene (0.34–4.36%), caryophyllene oxide (0.82–50.43%), humulene epoxide II (1.21–5.67%), δ-cadinol (1.10–7.92%), epi-γ-eudesmol (3.03–10.83%), globulol (0.82–42.28%), viridiflorol (2.34–39.14%), β-elemene (0.47–18.62%), shyobunol (1.37–7.5%) and t-cadinol (1.29–4.46%). The volatiles with high area coverage were juniper camphor (70.85%), caryophyllene oxide (50.43%), globulol (42.28%), viridiflorol (39.14%), α-pinene (36.56%), and β-selinene (20.53%). The physicochemical parameters of fixed oils of kernel and mace of H. amygdalina, H. kingii, K. angustifolia, K. linifolia and K. tenuinervia for the first time showed the yield 6.10–44.35% with compositional abundance of myristic acid, methyl ester (20.87–86.1%), lauric acid, methyl ester (35.81–40.02%), oleic acid, methyl ester (30.2–47.45%), palmitic acid, methyl ester (19.16–37.9%), mostly the saturated fatty acids. Both essential and fixed oils of wild nutmegs depicted the commercial potential of wild biowaste for novel renewable source of biomarkers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical Systematics and Ecology
生物-进化生物学
CiteScore
3.00
自引率
12.50%
发文量
147
审稿时长
43 days
期刊介绍:
Biochemical Systematics and Ecology is devoted to the publication of original papers and reviews, both submitted and invited, in two subject areas: I) the application of biochemistry to problems relating to systematic biology of organisms (biochemical systematics); II) the role of biochemistry in interactions between organisms or between an organism and its environment (biochemical ecology).
In the Biochemical Systematics subject area, comparative studies of the distribution of (secondary) metabolites within a wider taxon (e.g. genus or family) are welcome. Comparative studies, encompassing multiple accessions of each of the taxa within their distribution are particularly encouraged. Welcome are also studies combining classical chemosystematic studies (such as comparative HPLC-MS or GC-MS investigations) with (macro-) molecular phylogenetic studies. Studies that involve the comparative use of compounds to help differentiate among species such as adulterants or substitutes that illustrate the applied use of chemosystematics are welcome. In contrast, studies solely employing macromolecular phylogenetic techniques (gene sequences, RAPD studies etc.) will be considered out of scope. Discouraged are manuscripts that report known or new compounds from a single source taxon without addressing a systematic hypothesis. Also considered out of scope are studies using outdated and hard to reproduce macromolecular techniques such as RAPDs in combination with standard chemosystematic techniques such as GC-FID and GC-MS.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


