铁催化的芳基氯化物铃木偶联反应
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
应用非常广泛的铃木双芳基偶联反应通常需要使用钯催化剂,但人们越来越希望用一种可持续发展性更强、成本更低的催化剂来替代这种金属,而铁催化剂则是一个特别有吸引力的目标。在这里,我们展示了一种带有 N-杂环碳烯配体的简单铁基催化剂,可用于有机锂试剂活化的芳基氯底物与芳基硼酸酯的铃木双芳基偶联反应,效果极佳。机理研究表明,Fe(I) 作为催化歧管的最低氧化态可能参与其中,并表明具有挑战性的步骤不是芳基氯底物的活化,而是反金属化步骤。这些发现很可能会使铁催化的碳-碳键形成转化与软亲核偶联伙伴的关系复兴。本文章由计算机程序翻译,如有差异,请以英文原文为准。

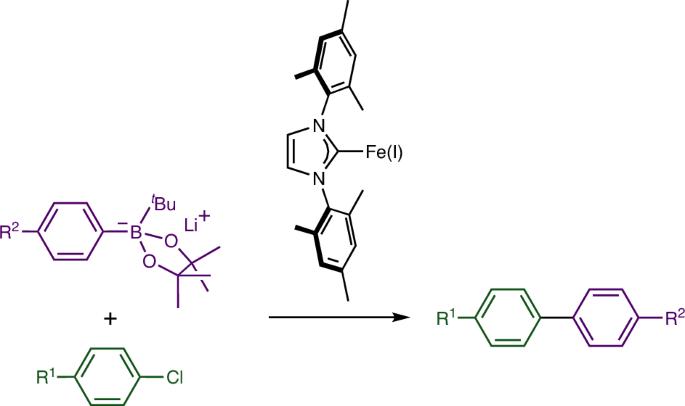
The iron-catalysed Suzuki coupling of aryl chlorides
The very widely exploited Suzuki biaryl coupling reaction typically requires catalysts based on palladium, but there is an increasing desire to replace this metal with a more sustainable, less expensive alternative, with catalysts based on iron being a particularly attractive target. Here we show that a simple iron-based catalyst with an N-heterocyclic carbene ligand can be used to excellent effect in the Suzuki biaryl coupling of aryl chloride substrates with aryl boronic esters activated by an organolithium reagent. Mechanistic studies suggest the possible involvement of Fe(I) as the lowest oxidation state on the catalytic manifold and show that the challenging step is not activation of the aryl chloride substrate, but rather the transmetallation step. These findings are likely to lead to a renaissance of iron-catalysed carbon–carbon bond-forming transformations with soft nucleophilic coupling partners. The replacement of palladium with other metal catalysts in C–C bond-forming reactions is attractive in terms of costs and sustainability. Now an iron-based catalyst is successfully employed in the Suzuki cross-coupling of aryl chlorides with aryl boronic esters activated with tert-butyl lithium.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


