苯胺类药物是蛋白质合成的特异性转运抑制剂
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
派尼拉米星是由蜜蜂病原体幼虫派尼巴氏菌(Paenibacillus larvae)产生的一组混合非核糖体肽-聚酮化合物,对金黄色葡萄球菌等革兰氏阳性病原体具有活性。虽然已证明苯胺类化合物能抑制蛋白质合成,但其作用机制仍不清楚。在这里,我们确定了苯氨基甲酰胺 PamB2-stalled 核糖体的结构,揭示了位于 A 位点和 P 位点转运核糖核酸(tRNA)之间的 30S 小亚基上的一个独特结合位点。除了精确描述 PamB2 与核糖体的相互作用外,这些结构还合理解释了幼虫 PamB2 的抗药性机制。我们进一步证明,PamB2 在翻译伸长过程中会干扰信使 RNA 和 tRNA 通过核糖体的转运,而且这种抑制活性会受到 A 位点 tRNA 第 37 位修饰的影响。总之,我们的研究将苯胺类化合物定义为一类特异性转位抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
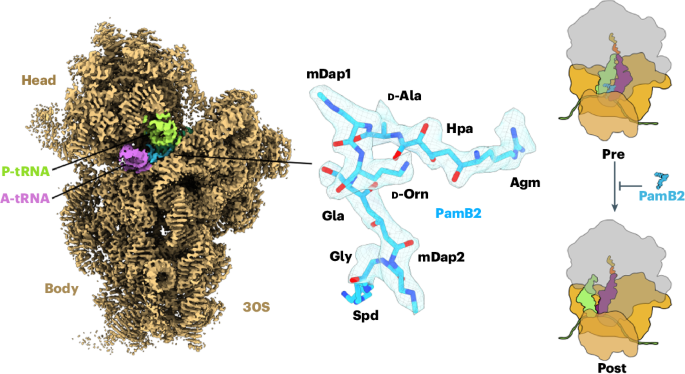
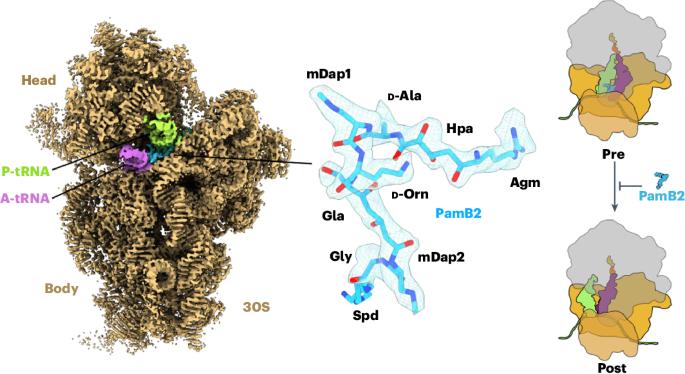
Paenilamicins are context-specific translocation inhibitors of protein synthesis
The paenilamicins are a group of hybrid nonribosomal peptide–polyketide compounds produced by the honey bee pathogen Paenibacillus larvae that display activity against Gram-positive pathogens, such as Staphylococcus aureus. While paenilamicins have been shown to inhibit protein synthesis, their mechanism of action has remained unclear. Here we determine structures of paenilamicin PamB2-stalled ribosomes, revealing a unique binding site on the small 30S subunit located between the A- and P-site transfer RNAs (tRNAs). In addition to providing a precise description of interactions of PamB2 with the ribosome, the structures also rationalize the resistance mechanisms used by P. larvae. We further demonstrate that PamB2 interferes with the translocation of messenger RNA and tRNAs through the ribosome during translation elongation, and that this inhibitory activity is influenced by the presence of modifications at position 37 of the A-site tRNA. Collectively, our study defines the paenilamicins as a class of context-specific translocation inhibitors. The paenilamicins are hybrid nonribosomal peptide–polyketide compounds that inhibit protein synthesis. Here the authors reveal that paenilamicins bind to a unique site on the ribosome, where they interfere with the translocation of mRNA and tRNAs during elongation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


