聚酰亚胺薄膜自组装循环弯曲胶原纤维
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
I 型胶原蛋白(Col)自组装形成的纤维的环状弯曲形态与各种疾病的发病机制密切相关。因此,在体外使 Col 分子自组装形成环形弯曲纤维的研究至关重要。在本研究中,我们通过成膜过程控制聚酰亚胺(PI)分子链的立体结构,成功地实现了 Col 分子的环状弯曲形状(特别是规则的六边形)。具体来说,在烘烤单层 PI 薄膜时,薄膜内的 PI 分子链沿平行于基底表面平面的方向弯曲。重复分层和烘烤过程后,PI 分子链形成三维结构,其方向与基底表面平面垂直。这种三维弯曲是上层和下层之间的 PI 分子链相互作用的结果。当 Col 分子在这些薄膜表面发生反应时,它们会识别 PI 分子链的结构,并自组装形成循环弯曲的 Col 纤维。特别是在经过三次分层和烘烤的 PI 薄膜中,可以观察到半圆形的 Col 纤维有规律地排列。此外,通过对 PI 薄膜进行单轴摩擦处理,这些规则循环弯曲的纤维在单轴方向上排列整齐。这项成功的研究意义重大,它既是一种控制 Col 纤维形态的方法,也是一项探索 Col 纤维在活体内循环弯曲的生物医学意义的研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
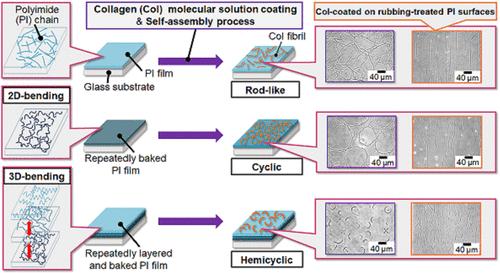
Self-Assembly of Cyclic-Bending Collagen Fibrils by Polyimide Films
The cyclic-bending morphologies of the fibrils formed by the self-assembly of type I collagen (Col) are closely related to the mechanisms of various diseases. Therefore, studies that allow the self-assembly of Col molecules to form cyclic-bending fibrils in vitro are vitally important. In this study, we successfully achieved the cyclic-bending shapes (specifically, a regular hexagonal shape) of Col molecules by controlling the steric structures of polyimide (PI) molecular chains through the film formation process. Specifically, when a single layer of PI film was baked, the PI molecular chains within the film bent in the direction parallel to the substrate surface plane. Repeating the layering and baking processes resulted in 3D structures of the PI molecular chains, which were oriented in the direction perpendicular to the substrate surface plane. This three-dimensional bending would result from the PI molecular chain interactions between the upper and lower layers. When the Col molecules were reacted on these film surfaces, they recognized the structures of the PI molecular chains and self-assembled to form cyclic-bending Col fibrils. Especially, in PI films subjected to three cycles of layering and baking, hemicircular-shaped Col fibrils were observed to be regularly arrayed. Additionally, these regularly cyclic-bending fibrils were aligned in the uniaxial direction through a uniaxial rubbing treatment of the PI films. This successful research is significant both as a method for controlling the morphologies of Col fibrils and as a study that explores the biomedical implications of Col fibril cyclic-bending in the living body.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


