鸟苷酸结合蛋白 GBP1 形成包裹细胞膜入侵细菌的蛋白外衣
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
GBP1 是一种重要的先天性免疫成分,有助于控制细胞膜侵入性细菌病原体。我们利用低温电子显微镜(cryo-EM)、低温电子断层扫描(cryo-ET)和生物物理实验,展示了 GBP1 寡聚体如何包裹和重塑革兰氏阴性细菌病原体的含脂多糖(LPS)膜。本文章由计算机程序翻译,如有差异,请以英文原文为准。
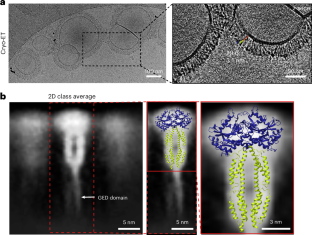

The guanylate-binding protein GBP1 forms a protein coat that enwraps cytosol-invasive bacteria
GBP1 is an important innate immunity component that contributes to the control of cytosol-invasive bacterial pathogens. Using cryo-electron microscopy (cryo-EM), cryo-electron tomography (cryo-ET) and biophysical assays, we show how GBP1 oligomers enwrap and remodel the lipopolysaccharide (LPS)-containing membrane of gram-negative bacterial pathogens.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


