液相铝纳米粒子及其作为铝/LiH 复合材料的吸氢性能
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在室温(25 °C)下,通过萘化二锂([Li2Naph])还原 AlH3,在甲苯中制备出纳米铝粒子 Al(0)。为了使起始材料溶解在甲苯中,AlH3 和 [Li2Naph] 被 N,N,N′,N′-四甲基乙二胺(TMEDA)配位。制备的 Al(0) 纳米粒子为单晶,大小均匀,为 11 ± 3 nm,粒度分布较窄。它们具有高活性,在空气中甚至与氮气都能发生反应。除了形成 Al(0) 纳米粒子外,[Li2Naph]驱动的 AlH3 还原还会形成 LiAlH4 副相,这种副相可以去除,但由于在不与 Al(0) 纳米粒子发生反应的溶剂(如甲苯、四氢呋喃)中溶解度低,很难去除。然而,LiAlH4 的存在以及 Al(0) 纳米粒子/LiAlH4 复合材料的形成在吸附 H2 方面具有优势。将 LiAlH4 热转变为 LiH(250 °C,真空)后,生成的 Al(0) 纳米粒子/LiH 复合材料在 250 °C、100 巴 H2 的条件下显示出良好的 H2 吸附性和 H2 吸收性,重量容量为 3.8%。用 1 wt % 的钯对纳米铝(0)颗粒进行活化改性后,甚至在 150 °C 的较低温度下也能吸收 H2。本文章由计算机程序翻译,如有差异,请以英文原文为准。
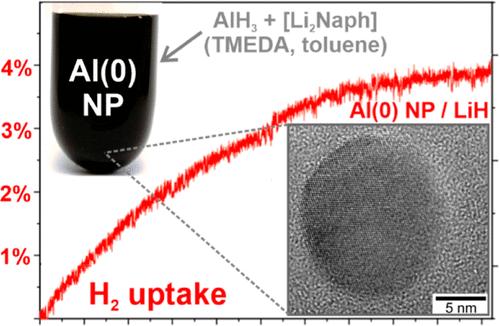
Liquid Phase-Made Aluminum Nanoparticles and Their Hydrogen Sorption as Aluminum/LiH Composite
Aluminum nanoparticles, Al(0), are prepared in toluene at room temperature (25 °C) by reduction of AlH3 with dilithium naphthalenide ([Li2Naph]). To dissolve the starting materials in toluene, AlH3 and [Li2Naph] are coordinated by N,N,N′,N′-tetramethylethylendiamin (TMEDA). The as-prepared Al(0) nanoparticles are monocrystalline and exhibit a uniform size of 11 ± 3 nm with narrow size distribution. They are highly reactive, as indicated by reaction in air and even with nitrogen. Besides the formation of Al(0) nanoparticles, the [Li2Naph]-driven reduction of AlH3 results in the formation of LiAlH4 as a side phase, which can be removed but is difficult to remove due to its low solubility in solvents that do not react with the Al(0) nanoparticles (e.g., toluene, THF). The presence of LiAlH4 and the formation of a Al(0) nanoparticle/LiAlH4 composite, however, turned out to be advantageous in regard to H2 sorption. After thermal transition of LiAlH4 to LiH (250 °C, vacuum), the resulting Al(0) nanoparticle/LiH composite shows promising H2 sorption and H2 uptake at 250 °C and 100 bar of H2 with a gravimetric capacity of 3.8%. After modification of the Al(0) nanoparticles with 1 wt % Pd for activation, the H2 uptake occurs at even a reduced temperature of 150 °C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


