通过对映选择性 Hofmann-Löffler-Freytag 反应制备手性吡咯烷酮
IF 11.6
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
自由基 C-H 亚胺化可以快速获得药物中最常见的杂环(如吡咯烷),但这些强大转化的立体控制仍然是一个挑战。在此,我们报告了首次发现的对映体和区域选择性 C-H 亚胺化反应,它能轻易地将酮转化为对映体富集的吡咯烷。这种对映选择性 Hofmann-Löffler-Freytag 反应机理是通过手性铜催化剂从肟中生成亚氨基自由基,促进 1,5-H 原子转移(HAT),从而选择性地形成一个远程 C-自由基。这种烷基自由基被有机铜(III)配合物选择性地捕获,然后介导高度立体选择性的还原消除反应,生成未受保护的吡咯烷。我们系统地探究了这种远程 C-H amination 的广泛立体和电子范围,以及对映体确定、自由基中间性和非典型 Cu(III)配体的关键机理方面,从而实现了这种独特的选择性 C-N 偶联。重要的是,对这些对映体富集的吡咯烷进行 (1) 还原或 (2) 亲核加成,提供了迄今为止最快速的手性吡咯烷合成方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
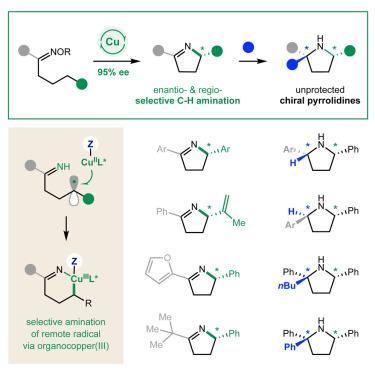
Chiral pyrrolidines via an enantioselective Hofmann-Löffler-Freytag reaction
Radical C–H aminations enable rapid access to the most common heterocycles in medicines (e.g., pyrrolidines), yet stereocontrol of these powerful transformations remains a challenge. Here, we report the discovery of the first enantio- and regioselective C–H imination, which readily converts ketones to enantioenriched pyrrolidines. This enantioselective Hofmann-Löffler-Freytag reaction mechanism entails iminyl radical generation from an oxime by a chiral Cu catalyst that facilitates 1,5-H-atom transfer (HAT) to form a remote C-radical regioselectively. The selective capture of this alkyl radical as an organocopper(III) complex then mediates highly stereoselective reductive elimination to unprotected pyrrolines. The broad steric and electronic scope of this remote C–H amination has been probed systematically, along with key mechanistic aspects of enantiodetermination, radical intermediacy, and atypical Cu(III) ligands that enable this uniquely selective C–N coupling. Importantly, either (1) reductions or (2) nucleophilic additions to these enantioenriched pyrrolines provide the most rapid syntheses of chiral pyrrolidines to date.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


