源自肠道共生菌的安乃近通过激活内源性大麻素途径促进蜜蜂的奖赏学习
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
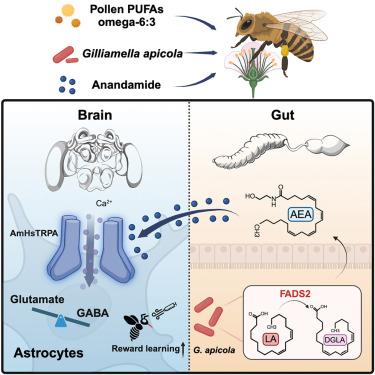
多不饱和脂肪酸(PUFA)是参与神经传递和细胞信号传导的膳食成分。花粉是蜜蜂的多不饱和脂肪酸来源之一,而膳食中的多不饱和脂肪酸会降低蜜蜂的认知能力。我们发现,蜜蜂的肠道细菌有助于脂肪酸代谢,从而影响奖励学习。肠道细菌拥有介导脂肪酸伸长的Δ-6去饱和酶,可弥补蜜蜂脂肪酸代谢所需因子的缺失。蜜蜂定殖 Gilliamella apicola(但不是缺乏 Δ-6 去饱和酶 FADS2 的突变体)会增加内源性大麻素系统的配体--anandamide(AEA)的产生,并改变学习和记忆。AEA能激活星形胶质细胞中的蝶呤特异性瞬时受体AmHsTRPA,从而诱导Ca2+流入并调节胶质细胞对谷氨酸的再摄取,从而提高奖赏学习能力。这些发现阐明了肠道共生体在宿主脂肪酸代谢中的作用以及内源性大麻素信号对社会性昆虫奖赏系统的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Gut symbiont-derived anandamide promotes reward learning in honeybees by activating the endocannabinoid pathway
Polyunsaturated fatty acids (PUFAs) are dietary components participating in neurotransmission and cell signaling. Pollen is a source of PUFAs for honeybees, and disruptions in dietary PUFAs reduce the cognitive performance of honeybees. We reveal that gut bacteria of honeybees contribute to fatty acid metabolism, impacting reward learning. Gut bacteria possess Δ-6 desaturases that mediate fatty acid elongation and compensate for the absence of honeybee factors required for fatty acid metabolism. Colonization with Gilliamella apicola, but not a mutant lacking the Δ-6 desaturase FADS2, increases the production of anandamide (AEA), a ligand of the endocannabinoid system, and alters learning and memory. AEA activates the Hymenoptera-specific transient receptor AmHsTRPA in astrocytes, which induces Ca2+ influx and regulates glutamate re-uptake of glial cells to enhance reward learning. These findings illuminate the roles of gut symbionts in host fatty acid metabolism and the impacts of endocannabinoid signaling on the reward system of social insects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


