用于感染性伤口护理的按需咪唑烷基脲基组织样自愈合抗菌水凝胶
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
细菌伤口感染是医疗保健领域面临的一个日益严峻的挑战,它带来了全身感染、器官衰竭和败血症等严重风险,预计到 2050 年,每年死亡人数将超过 1000 万。抗菌水凝胶具有类似细胞外基质的适应性,正在成为治疗感染性伤口的理想解决方案。然而,大多数此类水凝胶的抗菌特性主要归因于外在因子,其作用机制仍然鲜为人知。在此,我们首次引入改性咪唑烷基脲(IU)作为聚合物骨架,用于开发类组织抗菌水凝胶。所设计的水凝胶具有类似组织的机械特性、出色的抗冻性和快速自愈合能力。研究人员利用分子动力学(MD)模拟和密度泛函理论(DFT)计算,深入了解了 H 键和金属配体的配位程度,从而对水凝胶的性能进行了微调。体外研究表明,水凝胶对小鼠成纤维细胞、人类皮肤、肺部和红细胞具有良好的生物相容性,并具有潜在的伤口愈合能力。此外,水凝胶还具有良好的三维打印性和显著的抗菌活性,这归功于浓度依赖性 ROS 生成、氧化应激诱导以及随后的细菌膜破坏。此外,体外生物膜研究证实,开发的水凝胶能有效防止生物膜的形成。因此,这些仿组织水凝胶为加速伤口愈合同时控制细菌感染提供了一个前景广阔的有效平台,为未来的伤口护理带来了希望。本文章由计算机程序翻译,如有差异,请以英文原文为准。
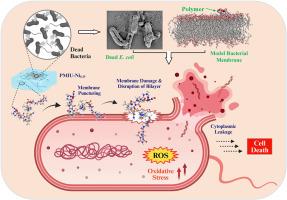
On-demand imidazolidinyl urea-based tissue-like, self-healable, and antibacterial hydrogels for infectious wound care
Bacterial wound infections are a growing challenge in healthcare, posing severe risks like systemic infection, organ failure, and sepsis, with projections predicting over 10 million deaths annually by 2050. Antibacterial hydrogels, with adaptable extracellular matrix-like features, are emerging as promising solutions for treating infectious wounds. However, the antibacterial properties of most of these hydrogels are largely attributed to extrinsic agents, and their mechanisms of action remain poorly understood. Herein we introduce for the first time, modified imidazolidinyl urea (IU) as the polymeric backbone for developing tissue-like antibacterial hydrogels. As-designed hydrogels behave tissue-like mechanical features, outstanding antifreeze behavior, and rapid self-healing capabilities. Molecular dynamics (MD) simulation and density functional theory (DFT) calculation were employed to well-understand the extent of H-bonding and metal-ligand coordination to finetune hydrogels’ properties. In vitro studies suggest good biocompatibility of hydrogels against mouse fibroblasts & human skin, lung, and red blood cells, with potential wound healing capacity. Additionally, the hydrogels exhibit good 3D printability and remarkable antibacterial activity, attributed to concentration dependent ROS generation, oxidative stress induction, and subsequent disruption of bacterial membrane. On top of that, in vitro biofilm studies confirmed that developed hydrogels are effective in preventing biofilm formation. Therefore, these tissue-mimetic hydrogels present a promising and effective platform for accelerating wound healing while simultaneously controlling bacterial infections, offering hope for the future of wound care.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


