用于微流体细胞拉伸测定的开源、电池供电、低成本双通道气动脉冲发生器
IF 2
Q3 ENGINEERING, ELECTRICAL & ELECTRONIC
引用次数: 0
摘要
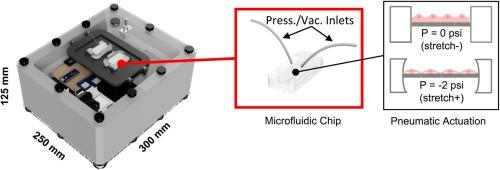
人体内的细胞经常受到机械力的作用,这些机械力会影响细胞的形态、基因表达和分化等生物学命运。目前在体外复制这些效应的黄金标准方法是在带有弹性基底的装置上培养细胞,并使用机械或气动拉推方法赋予细胞机械拉伸力。在这种情况下,微流控装置设计在一般的均匀和可控拉伸方面具有一些优势。然而,这些实验装置体积庞大、使用不便,而且往往涉及组织培养箱外的多个组件。鉴于机械刺激在体外研究中的广泛用途,我们的目标是创建一种交钥匙研究工具,生物工程人员可将其用于细胞拉伸实验,而不必处理临时实验装置的复杂性和细微差别。在这里,我们介绍一种开源、电池供电的双通道循环气动脉冲发生器箱,它可以放置在培养箱中,并与定制的微流体细胞拉伸装置兼容。我们的方法依赖于利用连续伺服电机驱动的线性微型气动气缸产生压力-真空脉冲。据我们所知,这是第一个完全由电池供电的独立系统实例,该系统在培养箱外没有任何外围设备。我们提供了不同组件的详细清单以及逐步组装过程。我们使用市售的微流控芯片在细胞拉伸试验中验证了它的性能。我们的结果表明,对人脐静脉内皮细胞(HUVECs)进行超过 8 小时的急性循环拉伸刺激会导致细胞优先排列成垂直于拉伸轴的直线。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An open-source, battery-powered, low-cost, and dual-channel pneumatic pulse generator for microfluidic cell-stretch assays
Cells in the body are regularly subjected to mechanical forces that influence their biological fate in terms of morphology, gene expression, and differentiation. The current gold standard method to replicate these effects in vitro is to culture cells on devices with elastic substrates and to impart mechanical stretch using mechanical or pneumatic pull–push methods. Microfluidic device designs offer several advantages in this context for general uniform and controlled stretching. However, the experimental setups are bulky, not user-friendly, and often involve several components that reside outside of the tissue culture incubator. Given the wide utility of mechanical stimulation in in-vitro research, our aim was to create a turn-key research tool that bioengineers can deploy in their cell-stretch assays, without having to deal with the complexity and nuances of ad hoc experimental setups. Here, we present an open-source, battery-powered, dual-channel cyclic pneumatic pulse generator box that can reside within an incubator and is compatible with custom microfluidic cell stretch devices. Our method depends on generating pressure-vacuum pulses simply using a linear miniature pneumatic air cylinder actuated using a continuous servo motor. To the best our knowledge, this is a first example of a completely battery-powered, standalone system that doesn’t have any peripherals residing out of the incubator. We provide a detailed list of different components as well as the step-by-step assembly process. We validate its performance in a cell stretch assay using a commercially available microfluidic chip. Our results show an acute stimulation of cyclic stretching over 8 h on human umbilical vein endothelial cells (HUVECs) resulted in preferential alignment of cells perpendicular to the axis of stretch.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

HardwareX
Engineering-Industrial and Manufacturing Engineering
CiteScore
4.10
自引率
18.20%
发文量
124
审稿时长
24 weeks
期刊介绍:
HardwareX is an open access journal established to promote free and open source designing, building and customizing of scientific infrastructure (hardware). HardwareX aims to recognize researchers for the time and effort in developing scientific infrastructure while providing end-users with sufficient information to replicate and validate the advances presented. HardwareX is open to input from all scientific, technological and medical disciplines. Scientific infrastructure will be interpreted in the broadest sense. Including hardware modifications to existing infrastructure, sensors and tools that perform measurements and other functions outside of the traditional lab setting (such as wearables, air/water quality sensors, and low cost alternatives to existing tools), and the creation of wholly new tools for either standard or novel laboratory tasks. Authors are encouraged to submit hardware developments that address all aspects of science, not only the final measurement, for example, enhancements in sample preparation and handling, user safety, and quality control. The use of distributed digital manufacturing strategies (e.g. 3-D printing) is encouraged. All designs must be submitted under an open hardware license.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


