不同 O2/N2 比率和加热速率下辣椒秸秆废物热解和燃烧特性的研究
IF 3.1
2区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
为了利用辣椒秸秆废弃物(CSW)生产能源并生成更高质量的燃料,我们使用热重分析-质谱法(TG-MS)研究了不同颗粒大小和加热速率下 CSW 的热解特性,以及不同 O2/N2 比率对其燃烧特性的影响。此外,还分析了三种不同条件下的产气性能。结果表明,固体产量随着粒径的增大而减少,粒径为 1.25 ∼ 1.60 毫米时的失重率最大,为 77.79%。加热速度为 10 K/min 时,相对热解率最高,为 0.77 %/K,相应的重量损失率为 74.46 %。大气中氧气比例的增加使 CSW 的点火温度和燃尽温度分别降低了 9.34 K 和 51.89 K,从而使衍生热重(DTG)曲线向更低的温度范围移动。此外,当加热速率从 5 K/min 提高到 40 K/min 时,H2 的峰值粒子电流从 8.7 × 10-10 A 上升到 1.2 × 10-9 A,增加了 0.38 倍。使用 Coats-Redfern (CR) 和 Achar 方法对 CSW 高温分解进行的动力学分析表明,活化能(Ea)随颗粒大小的增加而增加,这表明由于热传导阻力的存在,需要更高的能量。Friedman、Kissinger-Akahira-Sunose(KAS)和 Ozawa-Flynn-Wall (OFW)方法显示,随着转化率的增加,Ea 也在增加,这与半纤维素、纤维素和木质素的分解相对应。在燃烧过程中,氧气浓度对 Ea 有很大影响,挥发物和固定碳的 Ea 会升高,木质素在高温下的 Ea 也会升高。CR 模型和 Achar 模型的拟合度很高,证实了它们在描述 CSW 热解和燃烧方面的可靠性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
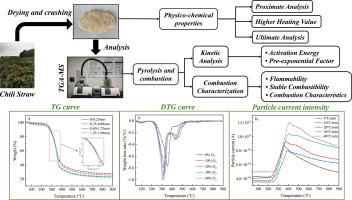
Investigation of pyrolysis and combustion characteristics of chili straw waste with different O2/N2 ratios and heating rates
To utilize chili straw waste (CSW) for energy production and generate higher-quality fuel, the pyrolysis characteristics of CSW under varying particle sizes and heating rates, as well as the effects of different O2/N2 ratios on its combustion characteristics, were investigated using Thermogravimetry-Mass Spectrometry (TG-MS). The gas production performance under three different conditions was also analyzed. Results indicated that the solid yield decreased as particle size increased, with the maximum weight loss rate of 77.79 % occurring at a particle size of 1.25∼1.60 mm. The highest relative pyrolysis rate was 0.77 %/K at a heating rate of 10 K/min, corresponding to a weight loss rate of 74.46 %. Increasing the proportion of oxygen in the atmosphere reduced both the ignition and burnout temperatures of CSW by 9.34 K and 51.89 K, respectively, shifting the Derivative Thermogravimetry (DTG) curve to a lower temperature range. Furthermore, an increase in the heating rate enhanced hydrogen production intensity during CSW pyrolysis, with the peak particle current of H2 rising from 8.7 × 10−10 A to 1.2 × 10−9 A, representing a 0.38-fold increase when the heating rate was raised from 5 K/min to 40 K/min. A kinetic analysis of CSW pyrolysis using the Coats-Redfern (CR) and Achar methods revealed that activation energy (Ea) increases with particle size, indicating higher energy requirements due to heat transfer resistance. The Friedman, Kissinger-Akahira-Sunose (KAS), and Ozawa-Flynn-Wall (OFW) methods showed rising Ea with increasing conversion rates, corresponding to the decomposition of hemicellulose, cellulose, and lignin. In combustion, oxygen concentration significantly influences Ea, raising it for volatile matter and fixed carbon, and also increasing it for lignin at high temperatures. The CR and Achar models provided strong fits, confirming their reliability in describing CSW pyrolysis and combustion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Thermochimica Acta
化学-分析化学
CiteScore
6.50
自引率
8.60%
发文量
210
审稿时长
40 days
期刊介绍:
Thermochimica Acta publishes original research contributions covering all aspects of thermoanalytical and calorimetric methods and their application to experimental chemistry, physics, biology and engineering. The journal aims to span the whole range from fundamental research to practical application.
The journal focuses on the research that advances physical and analytical science of thermal phenomena. Therefore, the manuscripts are expected to provide important insights into the thermal phenomena studied or to propose significant improvements of analytical or computational techniques employed in thermal studies. Manuscripts that report the results of routine thermal measurements are not suitable for publication in Thermochimica Acta.
The journal particularly welcomes papers from newly emerging areas as well as from the traditional strength areas:
- New and improved instrumentation and methods
- Thermal properties and behavior of materials
- Kinetics of thermally stimulated processes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


