Pd 支持的掺锰 NiO 催化剂的低温甲烷氧化能力增强
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
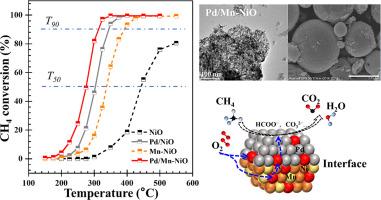
本研究介绍了一种新型催化剂体系,该体系由负载在掺锰氧化镍上的钯纳米颗粒组成,用于在低温下高效氧化甲烷(CH4)。在掺杂锰的载体上负载钯纳米颗粒后,催化剂具有优异的活性和稳定性,其最低 T50 温度为 276 ℃,325 ℃ 时的甲烷(CH4)转化率达到 97%。该催化剂优化了反应动力学,活化能最低,为 69.2 kJ mol-1。该催化剂的优异性能归功于 Pd/Mn-NiO 复合材料的协同效应,其中包括较高的 Pd2+/Pd4+ 比率、较高的吸附氧浓度(Oads/Ototal)以及较低的 Ni3+/Ni2+ 比率。这些特性共同提高了 CH4 氧化的催化活性。Mars-van Krevelen 机制是氧化过程的基础,Pd2+ 被确定为吸附和解离 CH4 的主要活性位点。漫反射红外傅立叶变换光谱与质谱联用技术阐明了表面活性氧物种形成甲酸盐等关键中间产物的途径,并强调了 Mn-NiO 表面的钯修饰促进了碳酸盐的加速分解。这些发现标志着在开发环境和能源相关应用催化剂方面取得了重大进展,特别是在减少甲烷排放方面。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced low-temperature methane oxidation over Pd supported Mn-doped NiO catalyst
This study introduces a novel catalyst system comprising Pd nanoparticles loaded onto Mn-doped NiO for the efficient oxidation of methane (CH4) at low temperatures. The loading of Pd nanoparticles onto the Mn-doped support has yielded a catalyst with exceptional activity and stability, as evidenced by the lowest T50 temperature of 276 °C and a CH4 conversion reaching 97% at 325 °C. The catalyst demonstrates optimized reaction kinetics with the lowest activation energy of 69.2 kJ mol–1. The catalyst's superior performance is attributed to the synergistic effects of the Pd/Mn-NiO composite, which include a higher Pd2+/Pd4+ ratio, increased adsorptive oxygen concentration (Oads/Ototal), and a reduced Ni3+/Ni2+ ratio. These characteristics collectively enhance the catalytic activity for CH4 oxidation. The Mars-van Krevelen mechanism underpins the oxidation process, with Pd2+ identified as the principal active site for CH4 adsorption and dissociation. Diffuse reflectance infrared Fourier transform spectroscopy coupled with mass spectrometry elucidates the pathway of surface reactive oxygen species in the formation of key intermediates, such as formates, and underscores the accelerated decomposition of carbonate facilitated by the Pd modification on the Mn-NiO surface. The findings signify a significant advancement in the development of catalysts for environmental and energy-related applications, particularly in the mitigation of CH4 emissions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


