揭示 ZrO2 表面苯酚直接羧化反应机理
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在当前的环境问题背景下,必须提出可持续的解决方案来处理废弃的二氧化碳这种众所周知的温室气体。在各种新兴项目中,将二氧化碳分子升级为高附加值化学品似乎很有前景。尤其是将芳香族化合物羧化为(二)酸性芳香族单体,对高性能聚合物行业具有重大意义。本文以苯酚直接羧化为对羟基苯甲酸的模型反应为重点,研究了 ZrO2 的反应活性。我们首次在金属氧化物材料表面建立了苯酚羧化机理,表明该反应只能通过 Eley-Rideal 机理进行。在这一机制中,二氧化碳在表面形成强烈的化学吸附,而苯酚则在靠近二氧化碳吸附物的地方形成物理吸附。此外,虽然单斜和四方相经常共存于 ZrO2 颗粒中,但我们证明只有单斜几何形状的 ZrO2 颗粒具有很高的活性。然而,选择性仍然是一个重大挑战,与最初的 Kolbe-Schmitt 方法一样,正交异构体是最丰富的产物。文献中报道的大多数苯酚直接羧化过程都是在液体介质中实现的,而描述固体表面羧化过程的理论知识却寥寥无几。因此,我们希望本手稿能成为一项开创性的工作,旨在更好地了解金属氧化物表面的反应性,为合理设计芳烃羧化反应的高效固体催化剂铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
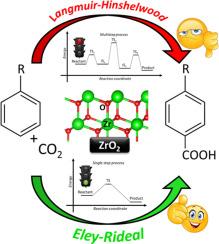
Unveiling the phenol direct carboxylation reaction mechanism at ZrO2 surface
In the present context of environmental concerns, sustainable solutions must be proposed to dispose of waste CO2, a well-known greenhouse gas. Among the various emerging projects, upgrading CO2 molecule into high-value added chemicals appears to be very promising. More particularly, the carboxylation of aromatic compounds to (di-) acid aromatic monomers is of great interest for the high performance polymer industry. Focusing on the direct phenol carboxylation to para-hydroxybenzoic acid as a model reaction, the reactivity of ZrO2 was investigated in this paper, this material being recently reported in various experimental works for its catalytic efficiency. For the first time, we established the phenol carboxylation mechanism at the surface of a metal oxide material, showing that the reaction can only proceed through an Eley-Rideal mechanism. In this mechanism, CO2 is strongly chemisorbed at the surface, whereas phenol is physisorbed close to the CO2 adsorbate. Besides, while the monoclinic and the tetragonal phases often coexist in ZrO2 particles, we demonstrated that only the monoclinic geometry exhibits a substantial activity. However, the selectivity remains a major challenge, the ortho- isomer being the most abundant product, as in the original Kolbe-Schmitt method. While most of the processes generally reported in literature for the direct carboxylation of phenol are achieved in liquid media, a very few theoretical knowledge is available to describe such a process at solid surfaces. Therefore, we expect the present manuscript to be a pioneer work, aiming at providing a better understanding of metal oxide surface reactivity, paving the road to the rational design of efficient solid catalysts for aromatics carboxylation reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


