可见光驱动的偶氮亚胺与羧酸的脱羧烷基化反应
IF 1.8
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
使用由 9-芳基吖啶和四丁基仲钨酸铵组成的双重催化体系,在 400 纳米光照射下,环状五元氮甲基亚胺与羧酸发生烷基化反应。含有芳香族、杂环族和脂肪族基团的偶氮亚胺适用于该工艺,可提供 42-98% 产率的吡唑烷-3-酮。本文章由计算机程序翻译,如有差异,请以英文原文为准。
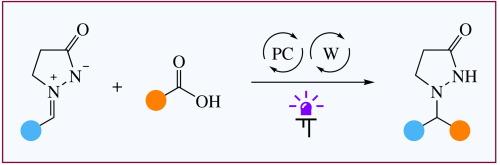
Visible light-driven decarboxylative alkylation of azomethine imines with carboxylic acids
The alkylation of cyclic five-membered azomethine imines with carboxylic acids proceeds under irradiation with 400 nm light using a dual catalytic system consisting of 9-arylacridine and tetrabutylammonium decatungstate. Azomethine imines containing aromatic, heterocyclic and aliphatic groups are suitable for the process providing pyrazolidin-3-ones in 42–98% yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


