在聚(ɛ-己内酯)嵌段中明确加入邻硝基苄基单元的光和 pH 双刺激响应嵌段共聚物胶束用于控释
IF 5.8
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
我们合成了一系列双刺激响应嵌段共聚物,它们的疏水性聚(ɛ-己内酯)嵌段中悬垂有不同含量的可光裂解邻硝基苄基(ONB)酯基,而在与亲水性聚(乙二醇)嵌段的连接处则存在可通过 pH 值裂解的缩醛连接。疏水嵌段是由ɛ-己内酯和 ONB 取代的ɛ-己内酯开环共聚合成的无规共聚物,两种单体的成分各不相同。聚合动力学表明,ONB 功能化单体的反应活性低于未取代单体。该系列嵌段共聚物在水溶液中能自组装成平均尺寸为 150-200 纳米的界限分明的球形胶束。通过核磁共振和紫外-可见光谱研究了 ONB 基团的光解作用,并确定了其程度。紫外线和 pH 值这两种刺激既可单独使用,也可同时使用,用于研究封装药物喜树碱的控制释放,并证明了这两种刺激的协同效应。观察了不同含量的 ONB 组对药物释放曲线的影响。MTT 试验表明聚合物无细胞毒性。实验证明了 MDA-MB-231 细胞对多柔比星(DOX)的吸收以及光诱导胶束对多柔比星(DOX)的释放。本文章由计算机程序翻译,如有差异,请以英文原文为准。
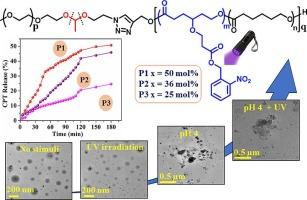
Photo and pH dual stimuli-responsive block copolymer micelles with defined incorporation of o-nitrobenzyl units in poly(ɛ-caprolactone) block for controlled release
A series of dual stimuli-responsive block copolymers with varying content of photocleavable o-nitrobenzyl (ONB) ester group pendent in the hydrophobic poly(ɛ-caprolactone) block and pH-cleavable acetal linkage at the junction with hydrophilic poly(ethylene glycol) block is synthesized. The hydrophobic block is a random copolymer synthesized by ring-opening copolymerization of ɛ-caprolactone and ONB-substituted ɛ-caprolactone containing varying compositions of the two monomers. Kinetics of polymerization shows that ONB-functionalized monomer has lower reactivity than that of the unsubstituted monomer. The series of block copolymers shows self-assembly into well-defined spherical micelles of average size of 150–200 nm in aqueous solution. Photocleavage of ONB groups is studied by NMR and UV–vis spectroscopy, and its extent is determined. The two stimuli viz. UV light and pH are used individually as well as simultaneously to study the controlled release of the encapsulated drug Camptothecin and the synergistic effect of the two stimuli is demonstrated. The effect of varying content of ONB groups is observed on drug release profile. MTT assay showed non-cytotoxic nature of the polymer. Cell uptake and photoinduced release of doxorubicin (DOX) from the micelles in MDA-MB-231 cells is demonstrated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


