新合成的苯并咪唑衍生物作为 1.0 M HCl 中低碳钢缓蚀剂的实验和计算研究:电化学、表面研究、DFT 建模和 MC 模拟
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
研究人员评估了两种新合成的苯并咪唑化合物 1-(环戊-1-烯-1-基)-3-(丙-2-炔-1-基)-1H-苯并咪唑-2(3H)-酮 (IMD1) 和 1-烯丙基-3-(环戊-1-烯-1-基)-1H-苯并咪唑-2(3H)-酮 (IMD2) 在 1.0 M HCl 溶液中对低碳钢 (MS) 的缓蚀作用。采用了电位极化(PDP)、电化学阻抗光谱(EIS)和失重(WL)分析等技术。EIS 分析表明,电阻随化合物浓度的增加而增加,这表明在 MS/HCl 界面形成了一层保护膜。通过 SEM-EDX 分析进一步证实了这种保护膜的形成。PDP 图显示了一种混合型抑制机制。在 10-4 M 浓度下,IMD1 和 IMD2 显示出显著的抑制效率,分别为 98.1 % 和 95.6 %。DFT 深入揭示了抑制剂分子与金属表面之间的电荷共享(供体-受体)相互作用。蒙特卡罗模拟(MCS)证实了这些结果,表明所研究的分子几乎平行于铁(1 1 0)表面吸附。本文章由计算机程序翻译,如有差异,请以英文原文为准。
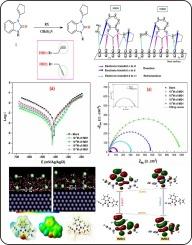
Experimental and computational study of newly synthesized benzimidazole derivatives as corrosion inhibitors for mild steel in 1.0 M HCl: Electrochemical, surface studies, DFT modeling, and MC simulation
Two newly synthesized benzimidazole compounds, 1-(Cyclopent-1-en-1-yl)-3-(prop-2-yn-1-yl)-1H-benzimidazol-2(3H)-one (IMD1) and 1-allyl-3-(cyclopent-1-en-1-yl)-1H-benzimidazol-2(3H)-one (IMD2), were evaluated for corrosion inhibition on mild steel (MS) in 1.0 M HCl solution. Techniques such as potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS), and weight loss (WL) analysis were employed. EIS analysis indicated increased resistance with compound concentration, suggesting the formation of a protective film at the MS/HCl interface. The formation of this protective coating was further confirmed through SEM-EDX analysis. PDP plots suggested a mixed-type inhibition mechanism. At 10−4 M concentration, IMD1 and IMD2 showed significant inhibition efficiencies of 98.1 % and 95.6 %, respectively. DFT gives insights into charge-sharing (donor–acceptor) interactions between inhibitor molecules and metallic surfaces. Monte Carlo simulation (MCS) confirmed these results, indicating that the molecules studied adsorbed almost parallel to the Fe (1 1 0) surface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


