Na2SO4 辅助下酸性镀液中铜电结晶成核机制的转变
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
本研究探讨了硫酸钠(Na2SO4)对电沉积过程中铜薄膜成核机制的影响。研究采用了伏安法和计时器法来分析电化学成核的动力学。通过 X 射线衍射对成核铜进行了相分析,同时使用扫描电子显微镜(SEM)和原子力显微镜(AFM)进行了形态和形貌研究,以进一步验证电化学研究结果。与未加入 Na2SO4 的沉积物相比,加入 Na2SO4 的沉积物更细腻、更紧密。此外,两种条件下沉积物的粗糙度也不同,有 Na2SO4 存在时形成的薄膜略微光滑。结果表明,Na2SO4 的加入改变了成核机制,从电荷转移控制转变为质量转移控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
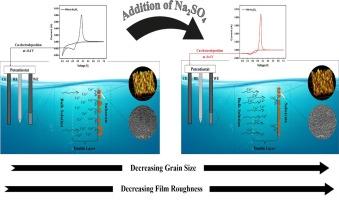
Na2SO4-assisted shift in the nucleation mechanism of copper-electrocrystallization from an acidic bath
In this study, the effect of sodium sulphate (Na2SO4) on the nucleation mechanism of copper films during electrodeposition was investigated. Voltammetric and chronoamperometric studies were employed to analyze the kinetics of electrochemical nucleation. Phase analysis of the nucleated copper was performed via X-ray diffraction, while morphological and topographical studies were conducted using scanning electron microscopy (SEM) and atomic force microscopy (AFM) to further validate the electrochemical findings. The deposits obtained in the presence of Na2SO4 were finer and more closely packed compared to those formed without Na2SO4. Additionally, the roughness of the deposits varied between the two conditions, with films formed in the presence of Na2SO4 being slightly smoother. The results indicate that the addition of Na2SO4 changed the nucleation mechanism from charge transfer controlled to mass transfer controlled.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


