制备 rGO/CAU-10 锚定钯铋复合材料及其乙二醇电催化性能
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
通过新型载体材料和多金属合金建立强界面相互作用,是提高电催化醇氧化催化性能的有效方法。本文制备了新型多面体金属有机框架铝基[Al(OH)(mBDC)](CAU-10)复合材料,其PdPbBi三金属粒子嵌入折叠氧化石墨烯(rGO)中,并通过简单的水热反应进行修饰,将其应用于乙二醇的电催化氧化反应。电化学测试结果表明,与商用 Pd/C 相比,三金属催化剂具有优异的电催化活性。其中,PdPbBi@rGO/CAU-10 的峰值电流密度最高,为 241.82 mA cm-2,是 Pd/C (26.92 mA cm-2)的 8.97 倍;电化学活性面积(ECSA)值最大,为 88.12 mA cm-2,是 Pd/C (36.75 mA cm-2)的 2.40 倍。这种突出的电催化活性主要得益于CAU-10复合载体的多面体结构和含氧原子的丰富性,有利于金属的均匀负载,同时Pd、Pb和Bi之间的强电子效应和含氧物种的丰富性也显著增强了电催化活性和稳定性,为乙二醇燃料电池电催化剂的开发提供了重要参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
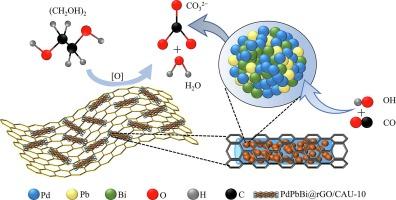
Preparation of rGO/CAU-10 anchored PdPbBi composites and their electrocatalytic ethylene glycol properties
An effective method to improve the catalytic performance of electrocatalytic alcohol oxidation by establishing strong interfacial interactions through novel carrier materials and polymetallic alloys. In this paper, novel polyhedral metal–organic framework Al-based [Al(OH)(mBDC)] (CAU-10) composites with PdPbBi trimetallic particles embedded in folded graphene oxide (rGO) modified by a simple hydrothermal reaction were prepared and applied to the electrocatalytic oxidation reaction of ethylene glycol. The results of electrochemical tests showed that the trimetallic catalysts exhibited excellent electrocatalytic activity compared to commercial Pd/C. In particular, PdPbBi@rGO/CAU-10 has the highest peak current density of 241.82 mA cm−2, which is 8.97 times higher than that of Pd/C (26.92 mA cm−2), and the largest electrochemical active area (ECSA) value of 88.12 mA cm−2, which is 2.40 times higher than that of Pd/C (36.75 mA cm−2). This outstanding electrocatalytic activity is mainly attributed to the polyhedral structure of CAU-10 composite carriers and the abundance of oxygen-containing atoms, which is conducive to the homogeneous loading of metals, at the same time, the strong electronic effect between Pd, Pb and Bi and the abundance of oxygen-containing species provide significant enhancement of electrocatalytic activity and stability, which provides an important reference for the development of electrocatalysts for ethylene glycol fuel cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


