叶酸功能化壳聚糖-PLGA 纳米粒子:胰腺癌靶向递送叶酸的新方法
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
高效的靶向癌细胞给药系统对于提高癌症治疗效果至关重要。在这项研究中,我们开发了包覆叶酸共轭壳聚糖的 PLGA 纳米颗粒(osthole-PLGA-NPs/CS-FA),用于向癌细胞递送 osthole,并研究了其在 PANC-1 胰腺癌细胞系中的抑制和分子信号转导机制。场发射扫描电子显微镜(FESEM)显示,osthole-PLGA-NPs/CS-FA呈球形结构,大小分布均匀。动态光散射(DLS)分析表明,其平均粒度为 171.76 nm,分散指数为 0.26,表面电荷为 + 33.08 mV,表明其稳定性和分散均匀性。傅立叶变换红外光谱(FTIR)分析证实,奥斯特孔成功地融入了聚乳酸乙二醛(PLGA)纳米颗粒,封装效率高达 93.12%。这些理化特性表明,奥斯特孔能被细胞高效吸收并进行靶向递送。使用 ABTS 法评估了 osthole-PLGA-NPs/CS-FA 的抗氧化潜力,结果表明其抑制自由基的作用与浓度有关,IC50 值为 172.95 μg/mL。使用 MTT 试验评估了其抗癌特性,结果表明它对 PANC-1 细胞具有显著的浓度依赖性细胞毒性作用(IC50 = 31.2 μg/mL),对正常人包皮成纤维细胞(HFF)的影响极小。DAPI 染色和流式细胞仪分析证实,PANC-1 细胞凋亡的增加与浓度有关。纳米颗粒诱导了 Bax 的上调和 Bcl2 的下调,表明线粒体内在凋亡途径被激活。使用小鸡绒毛膜(CAM)试验评估了 osthole-PLGA-NPs/CS-FA 的抗血管生成活性。结果表明,从 40 μg/mL开始,到 120 μg/mL为止,该物质以浓度依赖的方式明显抑制了血管生成。总之,osthole-PLGA-NPs/CS-FA 纳米粒子通过增强细胞摄取、诱导细胞凋亡和抑制血管生成,在胰腺癌靶向治疗中展现出了巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
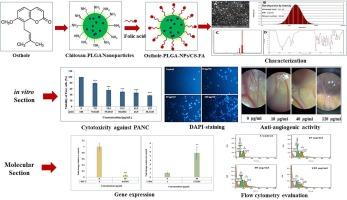
Folate-Functionalized Chitosan-PLGA Nanoparticles: A Novel approach for targeted osthole delivery in pancreatic cancer
Efficient drug delivery systems targeting cancer cells are crucial for enhancing cancer therapy. In this study, we developed PLGA nanoparticles coated with folate-conjugated chitosan (osthole-PLGA-NPs/CS-FA) to deliver osthole to cancer cells and investigated its inhibitory and molecular signaling mechanisms in the PANC-1 pancreatic cancer cell line. Field emission scanning electron microscopy (FESEM) revealed that osthole-PLGA-NPs/CS-FA had a spherical structure with a uniform size distribution. Dynamic light scattering (DLS) analysis showed an average size of 171.76 nm, a dispersion index 0.26, and a surface charge of + 33.08 mV, indicating stability and uniform dispersion. Fourier-transform infrared (FTIR) spectrum analysis confirmed the successful incorporation of osthole into the PLGA nanoparticles, with an encapsulation efficiency of 93.12 %. These physicochemical properties suggest efficient cellular uptake and targeted delivery. The antioxidant potential of osthole-PLGA-NPs/CS-FA was evaluated using the ABTS assay, showing concentration-dependent inhibition of free radicals with an IC50 value of 172.95 μg/mL. The anticancer properties were assessed using the MTT assay, demonstrating a significant and concentration-dependent cytotoxic effect on PANC-1 cells (IC50 = 31.2 μg/mL) with minimal impact on normal human foreskin fibroblast (HFF) cells. DAPI staining and flow cytometry analyses confirmed a concentration-dependent increase in apoptosis in PANC-1 cells. The nanoparticles induced upregulation of Bax and downregulation of Bcl2, indicating activation of the intrinsic mitochondrial apoptotic pathway. The anti-angiogenic activity of osthole-PLGA-NPs/CS-FA was evaluated using the chick chorioallantoic membrane (CAM) assay. The results showed significant inhibition of angiogenesis in a concentration-dependent manner, starting at 40 μg/mL and increasing up to 120 μg/mL. In conclusion, osthole-PLGA-NPs/CS-FA nanoparticles exhibit promising potential for targeted pancreatic cancer therapy by enhancing cellular uptake, inducing apoptosis, and inhibiting angiogenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


