先进的固态核磁共振技术揭示的葡萄糖水碳由连接的苯酚、呋喃、芳香族化合物、烷基和酮结构组成
IF 2.4
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
基于先进的一维和二维(2D)1H-13C 和 13C-13C 固态核磁共振(NMR)以及光谱编辑,我们阐明了在不同条件下从富含 13C 的葡萄糖制得的水合烃的分子结构。无论合成条件如何,水合碳主要由氧取代的炔环(包括二酚)和呋喃组成,并由富含酮的烷基连接体连接。交联的非质子化和甲基(C-H)烷基碳已通过光谱编辑二维核磁共振确定。只有在低合成温度下才能观察到烯烃和 "四元 "C-O,而在高温下会产生一些融合的炔环簇。13C 磁化在 1 秒内达到平衡,这表明材料在 5 纳米尺度上是均质的,反驳了水碳微球的核壳模型。与烷基或酮键合的呋喃 C-O 碳在二维核磁共振中显示出明显的交叉峰,而酚类 C-OH 则可通过羟基质子选择明确观察到。虽然亚甲基连接的呋喃环相当常见,但以前归因于呋喃 Cα-Cα 连接的信号被证明来自丰富、稳定的儿茶酚原二酚,其 HO-C=C-OH 结构在羟基质子选择后通过二维 13C-13C NMR 得到证实。低温和高温碳氢化合物的定量 13C NMR 光谱与代表性结构模型的化学位移模拟相匹配。由酮类和烷基连接物连接的混合酚环和呋喃环能很好地拟合实验光谱,而文献中以大簇融合环为主、含有少量酚类或烷基连接酮类的结构模型则不能很好地拟合实验光谱。本文章由计算机程序翻译,如有差异,请以英文原文为准。
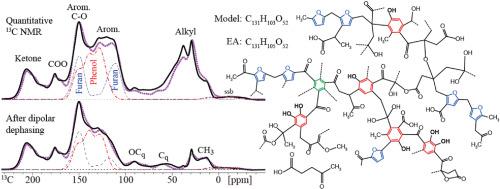
Glucose hydrochar consists of linked phenol, furan, arene, alkyl, and ketone structures revealed by advanced solid-state nuclear magnetic resonance
The molecular structure of hydrochars produced from 13C-enriched glucose under various conditions has been elucidated based on advanced one- and two-dimensional (2D) 1H-13C and 13C–13C solid-state nuclear magnetic resonance (NMR) with spectral editing. Regardless of synthesis conditions, hydrochars consist mostly of oxygen-substituted arene rings (including diphenols) and furans connected by alkyl linkers rich in ketones. Cross-linking nonprotonated and methyne (C-H) alkyl carbons have been identified through spectrally edited 2D NMR. Alkenes and ‘quaternary’ C-O are observed only at low synthesis temperature, while some clusters of fused arene rings are generated at high temperature. Hydrochar composition is nearly independent of reaction time in the range from 1 to 5 h. Equilibration of 13C magnetization within 1 s shows that the materials are homogeneous on the 5-nm scale, refuting core–shell models of hydrochar microspheres. While furan C-O carbons bonded to alkyl groups or ketones show distinctive cross peaks in 2D NMR, phenolic C-OH is observed unambiguously by hydroxyl-proton selection. While methylene-linked furan rings are fairly common, the signal previously assigned to furan Cα-Cα linkages is shown to arise from abundant, stable catecholic ortho-diphenols, whose HO-C=C-OH structure is proved by 2D13C–13C NMR after hydroxyl-proton selection. Quantitative 13C NMR spectra of low- and high-temperature hydrochars have been matched by chemical-shift simulations for representative structural models. Mixed phenol and furan rings connected by ketones and alkyl linkers provide good fits of the experimental spectra, while literature models dominated by large clusters of fused rings and with few phenols or alkyl-linked ketones do not.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.30
自引率
9.40%
发文量
42
审稿时长
72 days
期刊介绍:
The journal Solid State Nuclear Magnetic Resonance publishes original manuscripts of high scientific quality dealing with all experimental and theoretical aspects of solid state NMR. This includes advances in instrumentation, development of new experimental techniques and methodology, new theoretical insights, new data processing and simulation methods, and original applications of established or novel methods to scientific problems.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


