通过减轻肠道炎症和调节肠道菌群治疗溃疡性结肠炎的多功能 MnGA 纳米酶
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
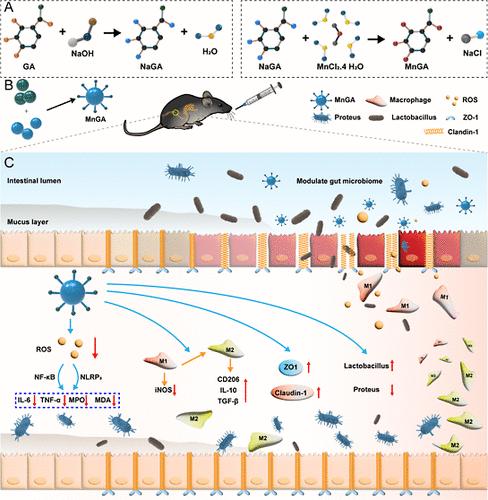
在溃疡性结肠炎(UC)中,炎症环境的形成是由于活性氧(ROS)和活性氮(RNS)产生过多、促炎细胞因子产生过多以及免疫系统功能紊乱等因素共同作用的结果。临床上治疗 UC 的传统药物种类繁多,但长期用药会产生毒副作用和耐药性,还会降低患者的依从性等弊端。因此,鉴于 UC 的临床难题,包括现有治疗方法的局限性、强烈的不良反应和耐药性的产生,迫切需要既能有效缓解症状又能保持高度生物安全性的新型治疗药物。尽管研究人员已开发出许多抗炎纳米药物,但开发高效无毒的纳米药物仍是临床医学的一大挑战。利用天然产物没食子酸和金属化合物氯化锰,开发出了一种用于治疗 UC 的高效无毒多功能纳米酶。纳米酶能有效消除 ROS 和 RNS,减轻氧化引起的肠上皮细胞炎症;通过上调紧密连接蛋白的表达,促进肠上皮屏障的恢复;平衡肠道微生物群,维持肠道环境的稳定。我们利用模仿 UC 的啮齿动物模型监测体重、结肠长度、脾脏指数和组织损伤程度,结果表明没食子酸锰(MnGA)纳米颗粒可以通过清除 ROS 和活性氮来减轻肠道炎症。肠道菌群测序显示,在 UC 小鼠模型中,MnGA 纳米粒子可以调节肠道菌群,促进有益菌的生长,降低肠道内有害菌的水平。因此,MnGA 纳米粒子可以维持肠道菌群的平衡。这项研究表明,MnGA 纳米粒子是一种优良的抗氧化剂和有效的抗炎剂,具有良好的生物安全性,可有效治疗 UC。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Multifunctional MnGA Nanozymes for the Treatment of Ulcerative Colitis by Reducing Intestinal Inflammation and Regulating the Intestinal Flora
In ulcerative colitis (UC), the formation of an inflammatory environment is due to the combined effects of excess production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), overproduction of proinflammatory cytokines, and disruption of immune system function. There are many kinds of traditional drugs for the clinical treatment of UC, but long-term drug use can cause toxic side effects and drug resistance and can also reduce patient compliance and other drawbacks. Hence, in light of the clinical challenges associated with UC, including the limitations of existing treatments, intense adverse reactions and the development of resistance to medications, no novel therapeutic agents that offer effective relief and maintain a high level of biosafety are urgently needed. Although many anti-inflammatory nanomedicines have been developed by researchers, the development of efficient and nontoxic nanomedicines is still a major challenge in clinical medicine. Using the natural product gallic acid and the metal compound manganese chloride, a highly effective and nontoxic multifunctional nanoenzyme was developed for the treatment of UC. Nanozymes can effectively eliminate ROS and RNS to reduce the inflammation of intestinal epithelial cells caused by oxidation, facilitate the restoration of the intestinal epithelial barrier through the upregulation of tight junction protein expression, and balance the intestinal microbiota to maintain the stability of the intestinal environment. Using a rodent model designed to mimic UC, we monitored body weight, colon length, the spleen index, and the degree of tissue damage and demonstrated that manganese gallate (MnGA) nanoparticles can reduce intestinal inflammation by clearing ROS and active nitrogen. Intestinal flora sequencing revealed that MnGA nanoparticles could regulate the intestinal flora, promote the growth of beneficial bacteria and decrease the levels of detrimental bacteria within the intestinal tract in a mouse model of UC. Thus, MnGA nanoparticles can maintain the balance of the intestinal flora. This study demonstrated that MnGA nanoparticles are excellent antioxidant and effective anti-inflammatory agents, have good biosafety, and can effectively treat UC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


