用于深层组织生物成像和治疗的 X 射线/γ 射线/超声波激活型持久发光荧光粉
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
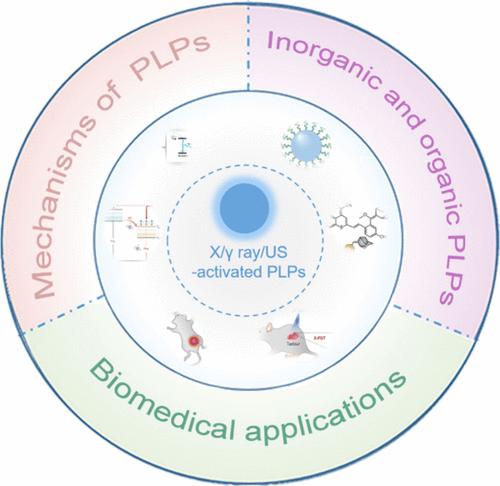
持久发光荧光粉(PLPs)可在激发停止后保持发光,自 2007 年以来已在生物成像和治疗领域得到广泛应用。在生物成像中,持久发光荧光粉可以在激发结束后收集持久发光信号,从而有效避免组织自发荧光和光散射干扰。利用 PLPs 进行生物成像可获得出色的信噪比、高灵敏度和高分辨率。在治疗方面,PLPs 可以在去除激发源后持续产生治疗分子(如活性氧),从而实现单剂量光刺激后的持续治疗活性。然而,大多数 PLPs 是由紫外线或可见光激活的,因此很难在体内重新激活 PLPs,尤其是在深层组织中。近年来,人们探索了具有深层组织穿透力的激发源来激活 PLPs,包括 X 射线、γ 射线和超声波。研究人员发现,X 射线、γ 射线和超声波可激活各种无机和有机聚磷酸酯,使这些聚磷酸酯在深部肿瘤的成像和治疗中发挥重要作用。在以往的综述中,还没有系统地介绍过这些被 X 射线/γ 射线/超声波激活的 PLPs。在这篇综述中,我们总结了最近开发的可被 X 射线、γ 射线和超声激活以产生持续发光的无机和有机 PLPs。我们还讨论了这些 PLPs 在深层组织生物成像和治疗中的生物医学应用。这篇综述可为设计具有深层组织可再生持续发光功能的 PLPs 提供指导,并进一步促进 PLPs 在光otheranostics、无创生物传感设备和能量收集方面的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
X-ray/γ-ray/Ultrasound-Activated Persistent Luminescence Phosphors for Deep Tissue Bioimaging and Therapy
Persistent luminescence phosphors (PLPs) can remain luminescent after excitation ceases and have been widely explored in bioimaging and therapy since 2007. In bioimaging, PLPs can efficiently avoid tissue autofluorescence and light scattering interference by collecting persistent luminescence signals after the end of excitation. Outstanding signal-to-background ratios, high sensitivity, and resolution have been achieved in bioimaging with PLPs. In therapy, PLPs can continuously produce therapeutic molecules such as reactive oxygen species after removing excitation sources, which realizes sustained therapeutic activity after a single dose of light stimulation. However, most PLPs are activated by ultraviolet or visible light, which makes it difficult to reactivate the PLPs in vivo, particularly in deep tissues. In recent years, excitation sources with deep tissue penetration have been explored to activate PLPs, including X-ray, γ-ray, and ultrasound. Researchers found that various inorganic and organic PLPs can be activated by X-ray, γ-ray, and ultrasound, making these PLPs valuable in the imaging and therapy of deep-seated tumors. These X-ray/γ-ray/ultrasound-activated PLPs have not been systematically introduced in previous reviews. In this review, we summarize the recently developed inorganic and organic PLPs that can be activated by X-ray, γ-ray, and ultrasound to produce persistent luminescence. The biomedical applications of these PLPs in deep-tissue bioimaging and therapy are also discussed. This review can provide instructions for the design of PLPs with deep-tissue-renewable persistent luminescence and further promote the applications of PLPs in phototheranostics, noninvasive biosensing devices, and energy harvesting.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


