用于有机光伏技术的氮桥接熔环非环和七环 A-D-A 受体
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
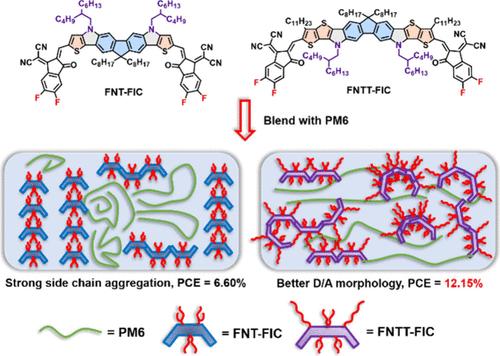
在这项工作中,我们设计了两种氮桥芴基七环 FNT 和非七环 FNTT 梯形结构,并通过一锅钯催化布赫瓦尔德-哈特维格胺化构建了这两种结构。FNT 和 FNTT 还被 FIC 受体进一步封端,分别形成两种 FNT-FIC 和 FNTT-FIC 非富勒烯受体(NFA)。与相应的碳杂化 FCT-FIC 和 FCTT-FIC 相比,这两种非富勒烯受体表现出更多的红移吸收和更高的结晶度。掠入射广角 X 射线散射(GIWAXS)测量结果表明,FNT-FIC 凸部氮上的 2-丁辛基基团与另一种 FNT-FIC 凹部芴上的二辛基基团相互咬合,形成了 d 间距为 13.27 Å 的片状堆积结构。因此,PM6:FNT-FIC(1:1 wt %)器件的功率转换效率(PCE)仅为 6.60%,这主要是由于 FNT-FIC 的高结晶性导致混合薄膜中的 PM6 和 FNT-FIC 出现明显的相分离。然而,FNTT-FIC 的 2-丁辛基基团位于其弧形骨架凹面区域内的氮上,可改善供体与受体的混溶性,从而促进形成更有利的形态。因此,PM6:FNTT-FIC(1:1.2 wt %)器件的 PCE 值达到了 12.15%,Voc 值达到了 0.96 V。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nitrogen-Bridged Fused-Ring Nonacyclic and Heptacyclic A–D–A Acceptors for Organic Photovoltaics
In this work, we designed two nitrogen-bridged fluorene-based heptacyclic FNT and nonacyclic FNTT ladder-type structures, which were constructed by one-pot palladium-catalyzed Buchwald–Hartwig amination. FNT and FNTT were further end-capped by FIC acceptors to form two FNT-FIC and FNTT-FIC non-fullerene acceptors (NFAs), respectively. The two NFAs exhibit more red-shifted absorption and higher crystallinity compared to those of the corresponding carbon-bridged FCT-FIC and FCTT-FIC counterparts. Grazing incidence wide-angle X-ray scattering (GIWAXS) measurements reveal that the 2-butyloctyl groups on the nitrogen in the convex region of FNT-FIC interdigitate with the dioctyl groups on the fluorene in the concave region of another FNT-FIC, resulting in a lamellar packing structure with a d spacing of 13.27 Å. As a consequence, the PM6:FNT-FIC (1:1 wt %) device achieved a power conversion efficiency (PCE) of only 6.60%, primarily due to the highly crystalline nature of FNT-FIC, which induced significant phase separation between PM6 and FNT-FIC in the blended film. However, FNTT-FIC, featuring 2-butyloctyl groups positioned on the nitrogen within the concave region of its curved skeleton, exhibits improved donor–acceptor miscibility, thereby promoting a more favorable morphology. As a result, the PM6:FNTT-FIC (1:1.2 wt %) device exhibited a higher PCE of 12.15% with an exceptional Voc of 0.96 V. This research demonstrates that placing alkylamino moieties within the concave region of curved A–D–A NFAs leads to a better molecular design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


