调节氢键网络的对称性供体-受体分子,用于改良金属锌阳极
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
活性水分子引起的锌枝晶和副反应问题严重影响了锌水电池(AZB)的发展。在此,一种名为 1,3-双(羟甲基)脲(BHMU)的对称氢键供体-受体分子添加剂可优先吸附在阳极表面,并通过氢键锁住水分子,从而隔离水分子,减少活性水分子引起的副反应。凭借这些优势,含有 BHMU 添加剂的混合电解质推动了具有高库仑效率(在 1 mA cm-2 下循环 1000 次以上,库仑效率为 99.7%)的可逆锌阳极的发展,同时还使对称电池在 1 mA cm-2 下稳定运行(1 mAh cm-2,6.更重要的是,Zn||PTO全电池在5 A g-1条件下连续循环3000次后,还能提供卓越的循环稳定性和更高的容量。这项研究对于利用对称性供体-受体分子调节水电解质中锌阳极的溶解结构和界面稳定性具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
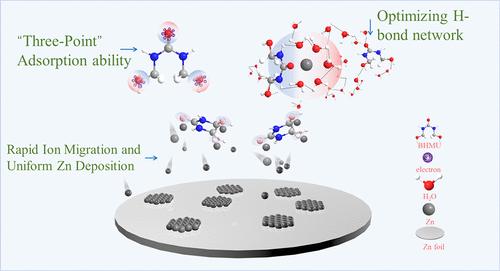
Symmetry Donor–Acceptor Molecule Regulating Hydrogen-Bonding Network for Improved Metal Zinc Anode
The issues of zinc dendrites and side reactions caused by active water molecules have seriously affected the development of aqueous zinc batteries (AZBs). Herein, a symmetry hydrogen-bond donor–acceptor molecule additive named 1,3-bis(hydroxymethyl)urea (BHMU) can preferentially adsorb on the anode surface and lock up water molecules through hydrogen bonding, thus isolating water molecules and reducing side reactions caused by active water molecules. With these advantages, the mixed electrolyte containing BHMU additive impels a reversible Zn anode with a high Coulombic efficiency (99.7% over 1000 cycles at 1 mA cm–2), while it also enables a stable symmetric cell operated at 1 mA cm–2 (1 mAh cm–2, 6.89% DODZn) for 2250 h and 10 mA cm–2 (10 mAh cm–2, 68.9% DODZn) for 350 h. More importantly, the Zn||PTO full battery also delivered superior cycling stability and higher capacity after 3000 consecutive 3000 cycles of circulation at 5 A g–1. This study has great significance for the use of symmetry donor–acceptor molecules to modulate the solvation structure and the interface stability of the Zn anode in aqueous electrolytes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


