基于细菌裂解物的双功能 mRNA 纳米制剂用于高效结肠癌免疫基因疗法
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
基于 mRNA 的非病毒基因疗法在癌症治疗中发挥了重要作用,但其有限的传递效率和治疗能力仍有待进一步探索和提高。免疫基因疗法为癌症治疗提供了一种策略。细菌是一种微小的单细胞生物,其中许多都存在于人体中或人体上,对人类有益。纽崔莱乳杆菌是肠道菌群中的一种细菌,最近的研究表明,它可以通过刺激免疫调节反应来减轻肠道炎症。L.reuteri裂解物是构建具有免疫刺激潜力的先进 mRNA 运送系统的理想资源。在此,我们制备了一种双功能mRNA递送系统DMP-Lac(DOTAP-mPEG-PCL-L. reuteri lysate),它成功地将L. reuteri lysate和IL-23A mRNA进行了编码递送,表现出75.56% ± 0.85%的高mRNA递送效率,并在体内强烈促进了免疫系统的成熟和激活。CT26腹腔转移模型和肺转移模型也显示出良好的治疗效果,DMP-Lac/IL-23A组的肿瘤抑制率达到97.92%。蛋白芯片技术验证了DMP作为免疫佐剂的作用,表明L.reuteri裂解物能调节相关免疫细胞,而IL-23 mRNA能引起下游因子的变化,从而产生相应的肿瘤治疗效果。DMP-Lac/IL-23A复合物具有很强的抗癌免疫治疗作用。我们的研究结果表明,这种双功能 mRNA 制剂可作为肿瘤特异性纳米药物,为结肠癌免疫基因治疗提供了一种先进的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
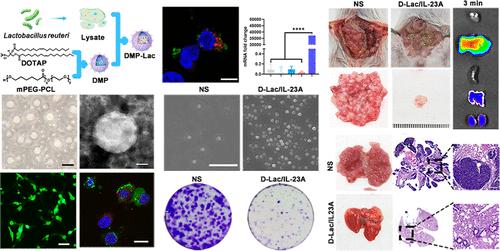
Bacterial Lysate–Based Bifunctional mRNA Nanoformulation for Efficient Colon Cancer Immunogene Therapy
mRNA-based nonviral gene therapy has played an important role in cancer therapy, however, the limited delivery efficiency and therapeutic capacity still require further exploration and enhancement. Immunogene therapy provides a strategy for cancer treatment. Bacteria are tiny single-celled living organisms, many of which can be found in and on the human body and are beneficial to humans. Lactobacillus reuteri is a bacterial member of the gut flora, and recent research has shown that it can reduce intestinal inflammation by stimulating an immunomodulatory response. L. reuteri lysate represents an ideal resource for constructing advanced mRNA delivery systems with immune stimulation potential. Here, we prepared a bifunctional mRNA delivery system DMP-Lac (DOTAP-mPEG–PCL-L. reuteri lysate), which successfully codelivered L. reuteri lysate and IL-23A mRNA, exhibited a high mRNA delivery efficiency of 75.56% ± 0.85%, and strongly promoted the maturation and activation of the immune system in vivo. Both the CT26 abdominal metastasis model and the lung metastasis model also exhibited a good therapeutic effect, and the tumor inhibition rate of DMP-Lac/IL-23A group reached 97.92%. Protein chip technology verified that DMP acted as an immune adjuvant, demonstrating that the L. reuteri lysate could regulate the related immune cells, while IL-23 mRNA caused changes in downstream factors, thus producing the corresponding tumor treatment effect. The DMP-Lac/IL-23A complex exhibited strong anticancer immunotherapeutic effects. Our results demonstrated that this bifunctional mRNA formulation served as a tumor-specific nanomedicine, providing an advanced strategy for colon cancer immunogene therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


