如何使心脏病学临床试验更具包容性
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
与人口疾病分布情况相比,心血管临床试验在儿童、老年人、女性和少数民族群体中的代表性仍然不足。在此,我们介绍了促进试验代表性的策略,并建议在试验资助、设计、开展和传播等层面采取行动。特别是,可以通过广泛的招募策略和选址标准来提高试验的代表性,这些策略和标准应反映出覆盖区患者的多样性,同时限制不合理的排除标准,并采用务实的设计,最大限度地减轻患者的研究负担(包括嵌入式和分散式试验)。试验交流应与文化相适应;让具有生活经验的不同人群参与某些试验要素的共同设计可促进文化相适应。试验人员本身的人口统计学特征与参与者的人口统计学特征相关联;因此,试验领导层必须积极多元化。资助机构和期刊越来越多地要求报告试验参与者的社会人口特征,监管机构现在也提供了关于提高试验多样性的指导;这些措施可能会增强变革的动力。尽管本视角侧重于心血管试验,但其中许多策略也可应用于其他领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
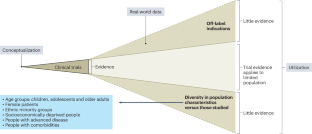
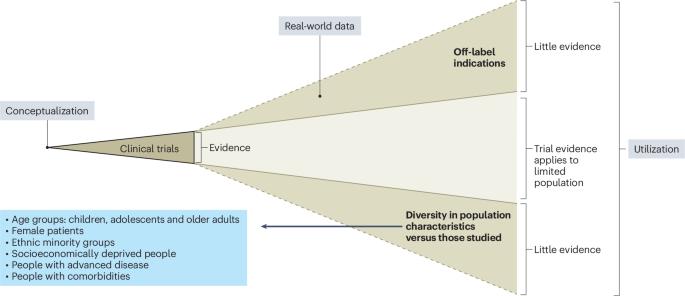
How to make cardiology clinical trials more inclusive
Cardiovascular clinical trials continue to under-represent children, older adults, females and people from ethnic minority groups relative to population disease distribution. Here we describe strategies to foster trial representativeness, with proposed actions at the levels of trial funding, design, conduct and dissemination. In particular, trial representativeness may be increased through broad recruitment strategies and site selection criteria that reflect the diversity of patients in the catchment area, as well as limiting unjustified exclusion criteria and using pragmatic designs that minimize research burden on patients (including embedded and decentralized trials). Trial communications ought to be culturally appropriate; engaging diverse people with lived experience in the co-design of some trial elements may foster this. The demographics of trialists themselves are associated with participant demographics; therefore, trial leadership must be actively diversified. Funding bodies and journals increasingly require the reporting of sociodemographic characteristics of trial participants, and regulatory bodies now provide guidance on increasing trial diversity; these steps may increase the momentum towards change. Although this Perspective focuses on the cardiovascular trial context, many of these strategies could be applied to other fields. Cardiology trials continue to under-represent certain population groups relative to disease distribution; this Perspective outlines strategies to foster representativeness and create a research enterprise that meets the needs of people living with cardiovascular disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


