金属钾还原芳基异氰酸酯和三芳基异氰酸酯形成的双芳基阴离子自由基
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
芳基异氰酸酯(芳基=苯基、对甲苯基、3,5-二甲基苯基、4-联苯基和 1-萘基)在 THF 中与 18-冠醚-6 或在 HMPA 中发生金属钾还原反应后,会形成相应的三芳基异氰脲酸酯阴离子自由基。继续与钾接触会导致异氰脲酸酯阴离子自由基的损失,最终形成相应的双芳基阴离子自由基。1,1′-联萘阴离子自由基会发生环氢化反应,从而形成过烯阴离子自由基。当正宗的三芳基异氰尿酸酯被金属还原时,杂环会随着一氧化碳的消除而发生破碎,生成三芳基双脲醛二阴离子。这种开环反应是由中性异氰尿酸盐的双电子还原反应引发的。双芳烷基阴离子在金属的作用下进一步还原,就形成了双芳烷基阴离子自由基。双芳基形成的一种可能机制是,一旦三芳基双脲酸酯二元离子被进一步还原,芳基 C-N 键就会发生异性裂解,并释放出芳基自由基。随后,两个芳基之间发生分子间反应,形成相应的双芳基,然后再还原成阴离子基。值得注意的是,当两种不同的三芳基异氰尿酸酯化合物的混合物在溶液中被还原时,就会生成相应的混合双芳基阴离子自由基。本文章由计算机程序翻译,如有差异,请以英文原文为准。
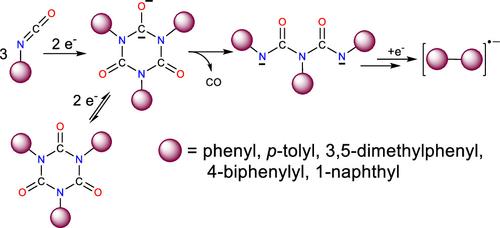
Biaryl Anion Radical Formation by Potassium Metal Reduction of Aryl Isocyanates and Triaryl Isocyanurates
The potassium metal reduction of aryl isocyanates (aryl = phenyl, p-tolyl, 3,5-dimethylphenyl, 4-biphenylyl, and 1-naphthyl) in THF with 18-crown-6 or in HMPA results in the formation of the corresponding triaryl isocyanurate anion radicals. Continued exposure to potassium results in loss of the isocyanurate anion radical and the eventual formation of the respective biaryl anion radical. The 1,1′-binaphthyl anion radical is found to undergo a cyclodehydrogenation reaction, which leads to formation of the perylene anion radical. When authentic triaryl isocyanurates are reduced with metal, the heterocyclic ring undergoes fragmentation with elimination of carbon monoxide to produce a triarylbiuret dianion. This ring opening reaction is initiated by the two-electron reduction of the neutral isocyanurate species. The biaryl anion radical is formed when the biuret dianion is reduced further with metal. A possible mechanism for biaryl formation involves a heterolytic cleavage of an aryl C–N bond and release of an aryl radical once the triarylbiuret dianion is further reduced. A subsequent intermolecular reaction between two aryl radicals forms the corresponding biaryl, which can then be reduced to the anion radical. Notably, when a mixture of two different triaryl isocyanurate compounds is reduced in solution, the corresponding mixed biaryl anion radical is generated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


