在各种复杂介质中对锂离子电池材料中的有毒金属进行高精度分析
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
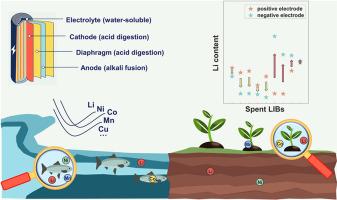
背景目前有关废锂电池管理和回收的法规不完善,这可能会导致在非正式处置此类电池时,水和土壤中的锂(Li)和重金属受到污染。要了解有毒金属在废锂电池和受污染的环境介质中的分布情况,对这些材料中的有毒金属采用精确的分析方法至关重要。然而,由于锂电池材料(如磷酸铁锂、石墨、隔膜和电解质)的化学性质复杂,目前仍缺乏针对各种环境介质中锂电池材料中有毒金属的分析技术的开发和验证研究。我们评估了不同 LIB 材料消化液的选择,并确定了质谱分析工作流程中最合适的内标元素。针对 LIB 所有成分设计的消解方案如下:王水消解所有阴极材料(不包括 LiMn0.6Fe0.4PO4 (LMFP));硝酸消解隔膜材料;王水加氢氟酸消解阳极材料 Li4Ti5O12 (LTO);NaOH 溶液消解石墨和 LMFP。LiPF6 电解液可直接溶解在超纯水中。通过采用这种方法,对不同环境基质(污水、土壤、植物、动物)中的锂离子电池阴极材料进行分析,可获得 83.6% 至 115.5% 的回收率。此外,该研究还揭示了锂和重金属在废锂电池阳极(石墨)中的显著积累。该方法为开展废锂电池环境风险评估提供了全面支持。利用这种方法,研究阐明了有毒金属在商用废锂电池不同部位的出现特征,为加强回收工作和促进废锂电池的风险管理提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。

High-precision analysis of toxic metals in lithium-ion battery materials across various complex media
Background
Present regulations regarding the management and recycling of spent Lithium-ion batteries (LIBs) are inadequate, which may lead to the pollution of lithium (Li) and heavy metals in water and soil during the informal disposal of such batteries. To comprehend the distribution of toxic metals within spent LIBs and contaminated environmental media, precise analytical methods for toxic metals in these materials are crucial. However, due to the chemical complexity of LIBs materials (e.g., lithium iron phosphate, graphite, separators, and electrolytes), there is still a lack of research on developing and validating analytical techniques for toxic metals in LIB materials across various environmental media.
Results
This study establishes a comprehensive and highly precise analytical method for assessing toxic metal constituents in LIBs across various complex media, including sewage, soil, and biological matrices. We assessed the selection of digestion solutions for different LIB materials and identified the most suitable internal standard elements in the mass spectrometric analysis workflow. The devised digestion schemes for all components of LIBs are as follows: aqua regia for all cathode materials (excluding LiMn0.6Fe0.4PO4 (LMFP)), nitric acid for diaphragm materials, aqua regia with hydrofluoric acid for the anode material Li4Ti5O12 (LTO), and NaOH fusion for graphite and LMFP. LiPF6 electrolyte can be directly dissolved in ultrapure water. By employing this method, the analysis of cathode materials of LIBs within diverse environmental matrices (sewage, soil, plants, animals) yields recovery rates ranging from 83.6 % to 115.5 %. Furthermore, this research reveals the remarkable accumulation of Li and heavy metals in anode (graphite) of spent LIBs.
Significance
This is the first to develop and validate analytical techniques for toxic metals in LIB materials across various environmental media, incorporating both acid digestion and alkaline fusion techniques. This methodology offers comprehensive support for conducting environmental risk assessments of spent LIBs. Using this method, the research elucidates the occurrence characteristics of toxic metals within different parts of commercial spent LIBs, providing valuable insights to enhance recycling efforts and facilitate risk management of spent LIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


